Preparative Separation of Polar Compounds using Atlantis T3 OBD Technology
LCGC Asia Pacific
Atlantis T3 HPLC columns are a universal silica-based, reversed-phase C18 columns that retain and separate highly polar compounds. The T3 bonding uses a trifunctional C18 alkyl phase bonded at a ligand density that promotes polar compound retention and aqueous mobile phase compatibility.
The Waters proprietary* Optimum Bed Density (OBD) design combines high-pressure slurry packing with a carefully calculated axial compression element localized at the less-dense inlet end of the bed. This establishes predictable, uniform density profiles throughout the column and results in exceptional resistance to mechanical chromatographic bed failure. In addition, the OBD design delivers consistent column-to-column performance, reducing cost through extended lifetimes.
The current work highlights the use of Atlantis T3 columns for the analytical and preparative separation of polar β-blockers. The initial separation was developed on a 4.6 × 100 mm, 5 μm analytical column, and subsequently scaled to both 19 mm and 30 mm i.d. columns. The ability to inject high mass loads of polar compounds corresponds to less preparative runs and faster library screening and purification.
Experimental
Figure 1
System: 2525 Binary Gradient Module, 2767 Sample Manager, 2996 PDA (AutoPurification flow cell)
Columns: Indicated on figure
Mobile phase: A: 0.1% TFA in water B: 0.1% TFA in ACN
Gradient: Initial hold at 0% B for 1 min, to 18% in 2.5 min, to 36% B in 5 min, hold at 100% B for 1 min, reset (13 min total cycle time)
Flow-rate: 25.59 mL/min
Injection: 1280 μL (44.8 mg total mass load)
Temperature: Ambient
Detection: UV @ 280 nm
Samples: Pyridoxine (5 mg/mL in H2O), acetaminophen (10 mg/mL in H2O), caffeine (20 mg/mL in H2O)
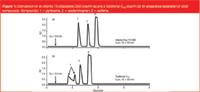
Figure 1
Figure 2
System: 2525 Binary Gradient Module, 2767 Sample Manager, 2996 PDA (AutoPurification flow cell)
Columns: Indicated on figure
Mobile phase: A: 0.1% formic acid in water B: 0.1% formic acid in ACN
Temperature: Ambient
Detection: UV @ 280 nm
Samples: Atenolol (40 mg/mL in DMSO), metropolol (200 mg/mL in DMSO), caffeine (200 mg/mL in DMSO)
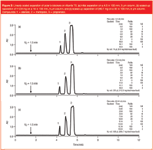
Figure 2
Gradient conditions, flow-rate and injection volume are indicated on the chromatograms.
Results and Discussion
Polar compounds present a unique and difficult challenge in preparative chromatography, because these analytes are not usually retained and/or poorly separated by reversed-phase (RP) HPLC. Figure 1 shows the preparative separation of a mixture of polar analytes on both the Atlantis Prep T3 OBD column and a traditional C18 column. Note that the retention of all compounds is much greater on Atlantis T3. In addition, the most polar compound (pyridoxine) exhibits split peak shape on the traditional C18 prep column because of its inability to retain pyridoxine properly. This problem is eliminated with the T3 prep column.
To demonstrate the linearity of scale-up between analytical and preparative dimensions, a separation of polar β-blockers was developed on a 4.6 × 100 mm, 5 μm Atlantis T3 column [Figure 2(a)]. Flow-rate, injection volume and gradient conditions were then scaled linearly to 19 × 100 mm, 5 μm [Figure 2(b)] and 30 × 100 mm, 5 μm columns [Figure 2(c)]. No resolution is lost between the analytical and preparative separations because all columns are packed with the same particle size sorbent. This example not only demonstrates that the Atlantis T3 columns can be operated at 100% aqueous starting conditions, but also that analytical scouting runs can accurately predict the chromatographic behaviour of polar compounds on the preparative scale.
Conclusions
An analytical method for the separation of polar b-blockers was developed and linearly scaled to the preparative dimension. Because the Atlantis T3 column was designed specifically for retention and separation of these types of compounds, it is capable of operating at 100% aqueous conditions. Just like their analytical equivalents, Atlantis T3 preparative OBD columns retain compounds longer. Therefore, stronger mobile phases and/or steeper gradient profiles can now be used, which results in more volatile peak fractions, faster fraction evaporation, less sample handling and higher recoveries.
*UK Patent Number: GB2408469
© 2007 Waters Corporation. Waters, The Science of What's Possible, Atlantis, OBD and AutoPurification are trademarks of Waters Corporation.
Kenneth J. Fountain, Steven M. Collier and Diane M. Diehl, Waters Corp., Milford, Massachusetts, USA.

Waters Corporation
34 Maple Street, Milford, Massachusetts 01757, USA
tel. +1 508 478 2000 fax +1 508 478 1990
Website: www.waters.com

Free Poster: NDSRI Risk Assessment and Trace-Level Analysis of N-Nitrosamines
April 25th 2025With increasing concern over genotoxic nitrosamine contaminants, regulatory bodies like the FDA and EMA have introduced strict guidelines following several high-profile drug recalls. This poster showcases a case study where LGC and Waters developed a UPLC/MS/MS method for quantifying trace levels of N-nitroso-sertraline in sertraline using Waters mass spectrometry and LGC reference standards.
New TRC Facility Accelerates Innovation and Delivery
April 25th 2025We’ve expanded our capabilities with a state-of-the-art, 200,000 sq ft TRC facility in Toronto, completed in 2024 and staffed by over 100 PhD- and MSc-level scientists. This investment enables the development of more innovative compounds, a broader catalogue and custom offering, and streamlined operations for faster delivery. • Our extensive range of over 100,000 high-quality research chemicals—including APIs, metabolites, and impurities in both native and stable isotope-labelled forms—provides essential tools for uncovering molecular disease mechanisms and exploring new opportunities for therapeutic intervention.
New Guide: Characterising Impurity Standards – What Defines “Good Enough?”
April 25th 2025Impurity reference standards (IRSs) are essential for accurately identifying and quantifying impurities in pharmaceutical development and manufacturing. Yet, with limited regulatory guidance on how much characterisation is truly required for different applications, selecting the right standard can be challenging. To help, LGC has developed a new interactive multimedia guide, packed with expert insights to support your decision-making and give you greater confidence when choosing the right IRS for your specific needs.
Using the Carcinogenic Potency Categorisation Approach (CPCA) to Classify N-nitrosamine Impurities
April 25th 2025Learn how to manage nitrosamine impurities in pharmaceuticals with our free infographic. Discover how the CPCA approach establishes acceptable intake limits and guides the selection of NDSRI reference samples. Stay compliant and ensure safety with our ISO-accredited standards.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)











