Rapid Analysis of Ginseng Using Accelerated Solvent Extraction and HPLC
LCGC Asia Pacific
Asian ginseng (Panax ginseng), American ginseng (Panax quinquefolius) and notoginseng (Panax notoginseng) are medicinal plants of the Araliaceae family, that studies have shown may improve mental performance and immunological response, increase blood circulation, reduce inflammation and alleviate pain.1,2 Ginsenosides are thought to be the active components that impart ginseng's medicinal properties. Sonication, hot reflux and soxhlet extraction are commonly used to extract ginsenosides, but can require from 2 to 14 h, are labour intensive and are often inconsistent.3 Accelerated solvent extraction (ASE) takes much less time (approximately 15 min) and uses less solvent while increasing efficiency.
Reported HPLC methods for ginsenoside analysis either require a long gradient (at least 60 min),2 or separate fewer individual ginsenosides in a single injection.4 The optimized HPLC method described here separates 15 ginsenosides within 25 min. This combined extraction/separation method is suitable for analysing Asian ginseng, American ginseng, and notoginseng.
Extraction Procedure
Powdered ginseng samples were placed in a Dionex ASE 200 accelerated solvent extractor and extracted using 100% methanol at 140 °C and 1500 psi with two 5 min static cycles. At the end of each static cycle the system was flushed with fresh solvent. Finally, the system was purged with nitrogen for 100 s. The extracts were diluted to 25 mL with DI water and filtered through 0.4 μm filters prior to injection. A second extraction using the optimized ASE method demonstrated that the extraction efficiency for all ginseng samples was >98%.
Chromatography Conditions
Analysis was performed using a Dionex UltiMate Intelligent LC system consisting of a HPG-3400 pump, WPS-3000 autosampler, TCC-3200 thermostatted column compartment and VWD-3400 variable wavelength detector. The system was controlled by Chromeleon Chromatography Management Software. Separation was performed using an Acclaim 120 C18 column (4.6 × 250 mm, 5 μm, P/N = 059149). For detailed chromatography conditions, please refer to Dionex Application Note 192.5
Results
Ginsenosides Re, Rg1 and the isomers Rb2 and Rb3 are difficult to separate by HPLC because they tend to coelute.6 Experiments showed that a good separation of ginsenosides Re and Rg1 could be achieved by increasing column temperature to 50 °C. Ginsenosides Rb2 and Rb3 were resolved using a simple gradient of acetonitrile. Fifteen ginsenosides, including the four ginsenosides discussed above, were separated in under 25 min using the specified chromatographic conditions.
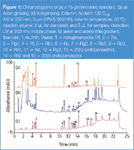
Figure 1
Ginseng samples from different species and countries of origin were analysed. The major ginsenosides in these samples were similar, but the ginseng varieties could be identified by their characteristic ginsenosides and the ratios of other ginsenosides.7 Figure 1 compares the chromatograms of notogensing, an Asian ginseng and a 15 ginsenoside standard. As the figure shows, this rapid extraction and analysis of ginsenosides can be used to identify ginseng varieties and to quantify ginsenoside levels for use in clinical studies.
Chen Jing, Xu Qun, Jeff Rohrer and Li Lang, Dionex Corp, Sunnyvale, California, USA.
References
1. Chinese Traditional Medicine; People's Medical Publishing House (2001).
2. Pharmacopoeia Commission of the Ministry of Public Health. Chinese Pharmacopoeia 2005, Part I. Chemical Industrial Press; Beijing, China (2005).
3. W. Vongsangnak et al., Biochem. Eng. J., 18(2), 115–120 (2004).
4. "Quick Separation of Active Ingredients from Gen-Seng", LaborPraxis China, 4, 18 (2006).
5. Dionex Corporation, Rapid Analysis of Ginseng Using Accelerated Solvent Extraction and High Performance Liquid Chromatography; Application Note 192, LPN 1965. Sunnyvale, California, USA (2007).
6. J.B. Wan et al., J. Pharm. Biomed. Anal., 41(1), 274–279 (2006).
7. W.M. Zhai et al., Chi. J. Chi. Mater. Med., 26(7), 481–482 (2001).
Acclaim, ASE, Chromeleon and UltiMate are registered trademarks of Dionex Corporation.

Dionex Corporation
1228 Titan Way, Sunnyvale, California 94085, USA
tel. +1 408 737 0700 fax +1 408 730 9403
Website: www.dionex.com

The Benefits of Custom Bonded Silica
April 1st 2025Not all chromatography resins are created equal. Off-the-shelf chromatography resins might not always meet the rigorous purification requirements of biopharmaceutical manufacturing. Custom bonded silica from Grace can address a wide range of separation challenges, leading to real performance improvements. Discover more about the latest innovations in chromatography silica from Grace, including VYDAC® and DAVISIL®.
5 Things to Consider When Selecting a Chromatography Silica
April 1st 2025Particularly in the pharmaceutical industry, drug purity isn’t just a goal – it’s essential for achieving safety, stability and efficacy. However, purification is easier said than done, especially with challenging molecules like DNA and RNA “oligonucleotides,” due in large part to their diversity and the range of impurities that can be generated during production. Enter DAVISIL® chromatographic silica, with a wide range of pore diameters and particle sizes to meet your specific application, performance and sustainability requirements. Before you choose the chromatography resin for your next purification application, take a look at these 5 considerations.
Automating Protein Purification: Efficiency, Yield, and Reproducibility
March 27th 2025Recent advancements in automated protein purification stress the importance of efficiency, scalability, and yield consistency. This eBook compares different purification platforms, highlighting their impact on downstream applications and demonstrating how automation enhances throughput and process control.
MilliporeSigma: Ultrapure Water for Sensitive LC-MS Analysis of Pesticides
March 25th 2025The aim of the study was to illustrate the efficiency of Milli-Q® water purification systems in eliminating pesticides from tap water, thereby producing and delivering reliable and consistent-quality ultrapure water suitable for pesticides analysis












