Accelerating Sample Preparation for the Analysis of Complex Samples
Sample preparation (that is, separation and enrichment) is a critical step in complex sample analysis that affects the sensitivity, selectivity, speed, and accuracy of analytical results, especially in rapid analysis. From chaos to order, the entropy reduction procedure of sample preparation cannot happen spontaneously. Given that sample preparation consumes over two thirds of analysis time, sample preparation becomes the bottleneck issue in analytical chemistry, resulting in the urgent necessity of developing accelerated sample preparation techniques.
Analytical chemistry faces the considerable challenge of how to accomplish the effective measurement of extremely complicated samples to satisfy increasing demands of fundamental research and practical application in areas such as life sciences, medicine, and food and environmental analysis. These samples are of many different types, are accompanied by complex matrices, and contain trace-level target analytes whose properties are incredibly similar to each other. Because of these conditions, excellent modern instrumental analysis methods, such as high performance liquid chromatography (HPLC), cannot deal with them directly without sufficient sample preparation methods.
Sample Preparation Strategies
Sample preparation enables separation and enrichment of target analytes from complex matrices, and thus can improve the sensitivity, selectivity, speed, and accuracy of analytical results. Unfortunately, the migration of target analytes from the original matrix to a more ordered pre-analysis state is an entropy reduction procedure, and does not happen spontaneously. As a result, sample preparation is often characterized by being time-consuming, labor-intensive, and error-prone, making it the bottleneck in the development of analytical methods. We must break through this bottleneck.
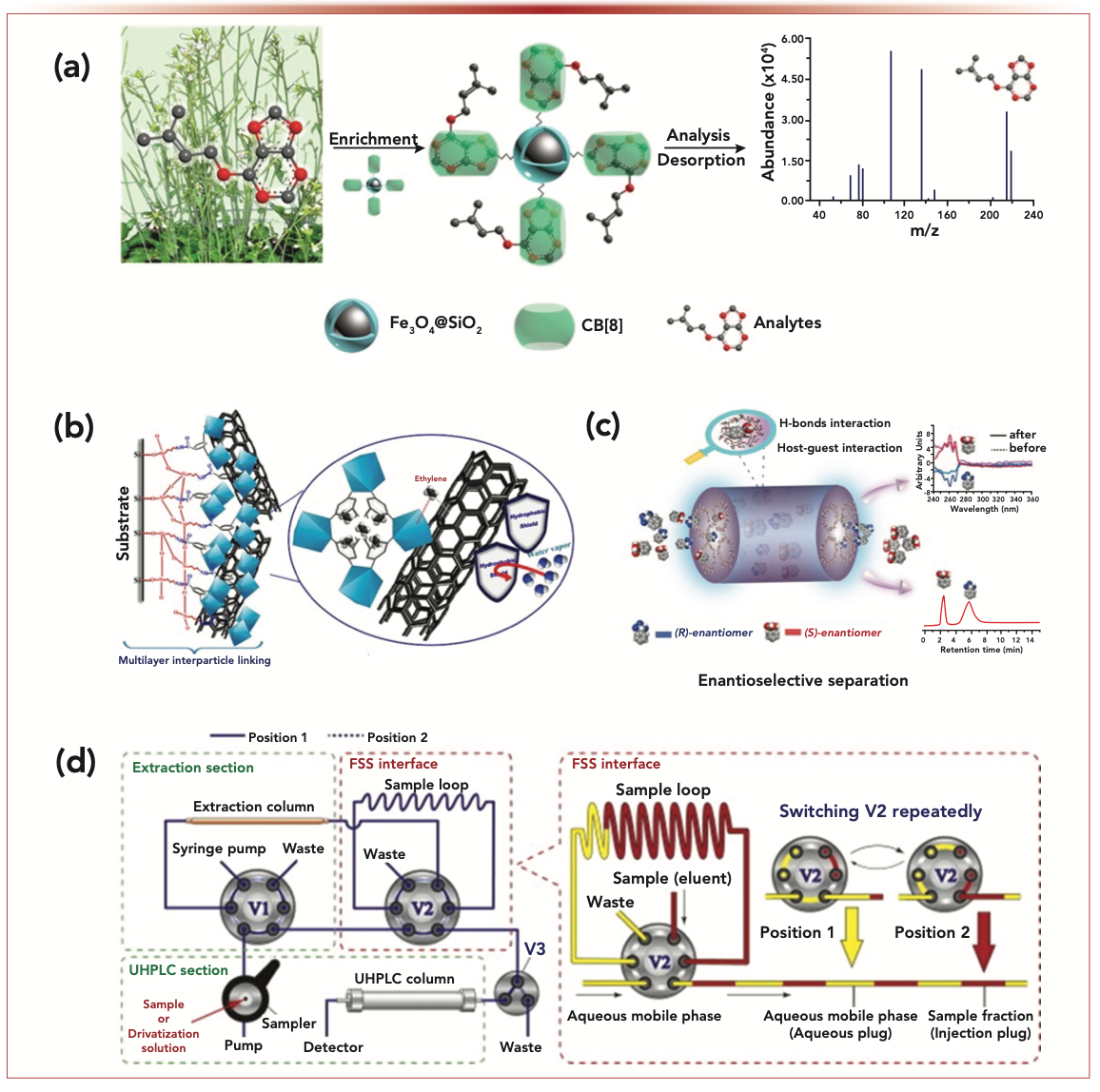
The development of accelerated sample preparation strategies, methods, materials, and devices has received much attention (1), especially in rapid analysis of complex samples. Field-assisted sample preparation techniques, which input energy, are a good match for rapid analysis. Various energy fields (including force, thermal, electrical, magnetic, acoustic, and microwave) have been utilized to expedite the mass transfer process for individual analytes, or simultaneously for multiple analytes. For example, magnetic field-assisted techniques can contribute to rapid separation, and they can also improve the sensitivity of the analytical method via efficient enrichment (2). In the analysis shown in Figure 1a, magnetic perhydroxy-cucurbit[8]url microspheres were fabricated to achieve rapid and selective enrichment of ultratrace cytokinins in plant samples (2). In another example, assisted by a hybrid field of microwave, ultrasonic, and magnetic fields, the extraction of organochlorine pesticide from tobacco was performed faster and more efficiently (3). Beyond inputting energy, introducing materials into the sample preparation process also contributes to rapidly achieving the pre-analysis state of the target analyte. These materials include (but are not limited to) graphene (4), metal-organic frameworks (5,6), covalent organic frameworks (7.8), and supramolecular porous polymers (9), which enable mass transfer or chemical transformation in a faster and effective manner, and are more suitable for rapid analysis. In addition, these introduced materials can contribute both as sample preparation media (see Figure 1b) and chromatographic packing materials (see Figure 1c).
FIGURE 1: (a) Efficient and selective enrichment of ultratrace cytokinins in plant samples by magnetic perhydroxy-cucurbit[8]uril microspheres (2). (b) Multilayer interparticle linking a hybrid metal-organic framework (MOF-199) for noninvasive enrichment and analysis of the plant hormone ethylene (5). (c) β-cyclodextrin porous polymers with three-dimensional chiral channels for separation of polar racemates (9). (d) A novel fractionized sampling and stacking strategy for online hyphenation of solid-phase extraction to ultrahigh-pressure liquid chromatography for ultrasensitive analysis (12).
In contrast to the approach of inputting energy or introducing materials during the sample preparation process, the size-reduction strategy reduces sample preparation time by dealing with a smaller amount of sample. Miniaturized sample preparation methods and devices are developing rapidly (10), such as solid-phase microextraction (SPME). Recent developments in microfluidics promote device miniaturization even further, making it possible to achieve picoliter-level sample preparation and analysis. More important, the introduction of additional energy and materials into microfluidics systems is easy. For example, by performing immunoreaction on the surface of magnetic beads and detection in a microfluidic device, magnetic materials and fields are employed, enabling highly effective biomarker analysis from as little as 10 μL of serum (11).
Integration Strategies
Integration strategies that involve combining procedures or materials in sequence or simultaneously have outstanding performance in reducing sample preparation time. Among these strategies, online coupling and high throughput approaches are commonly used. Through online coupling of multiple sample preparation and detection procedures, sample transfer time can be eliminated. Even better, the automated nature of online coupling strategies may improve the accuracy of analytical results (12,13). Figure 1d shows an example of online coupling of solid-phase extraction (SPE) and ultrahigh-pressure liquid chromatography (UHPLC) for trace analysis in a complex sample. By preparing numerous samples simultaneously, the total analysis time is shortened without procedure modification (14), an approach that is widely adopted in ‘omics studies. Taking this further, accomplishing several sample preparation and detection procedures in a single step is an emerging approach for rapid analysis. This all-in-one strategy has shown remarkable time-saving capacity in sample preparation for various analysis methods, including chromatography (15), chemiluminescence (16,17), and surface-enhanced Raman scattering (18).
Another integration strategy that should be mentioned is in situ sample analysis. By skipping the sample transfer step, this strategy not only saves time, but also maintains the original state and composition of the sample. The combination of all the acceleration strategies for sample preparation discussed above could speed up complex sample analysis even further, especially in chromatography (19).
Summary
The amount of time required for sample preparation is an obstacle for revolutionizing analytical methods. Taking HPLC as an example, over two thirds of the total analysis time is generally consumed by sample preparation. To speed up and achieve rapid analysis of complex samples, accelerated sample preparation strategies, methods, materials, and devices are urgently needed.
Acknowledgments
The author wishes to thank the State Key Program of National Natural Science of China (No. 22134007).
References
(1) L. Xia, J. Yang, R. Su, W. Zhou, Y. Zhang, Y. Zhong, S. Huang, Y. Chen, and G. Li, Anal. Chem. 92, 34–48 (2020).
(2) Q. Zhang, G. Li, X. Xiao, S. Zhan, and Y. Cao, Anal. Chem. 88, 4055–4062 (2016).
(3) T. Zhou, X. Xiao, and G. Li, Anal. Chem. 84, 420–427 (2012).
(4) S. Zhang, Z. Du, and G. Li, Anal. Chem. 83, 7531–7541 (2011).
(5) Z. Zhang, Y. Huang, W. Ding, and G. Li, Anal. Chem. 86, 3533–3540 (2014).
(6) Y. Hu, H. Lian, L. Zhou, and G. Li, Anal. Chem. 87, 406–412 (2015).
(7) Y. Chen, L. Xia, Z. Lu, G. Li, and Y. Hu, J. Chromatogr. A 1654, 462475 (2021).
(8) J. Pan, S. Jia, G. Li, and Y. Hu, Anal. Chem. 87, 3373–3381 (2015).
(9) Y. Chen, Z. Lu, G. Li, and Y. Hu, J. Chromatogr. A 1626, 461341 (2020).
(10) Z. Chen, G. Li, and Z. Zhang, Anal. Chem. 89, 9593–9600 (2017).
(11) J. Yang, X. Xiao, L. Xia, G. Li, and L. Shui, Anal. Chem. 93, 8273–8280 (2021).
(12) J. Pan, Y. Huang, L. Liu, Y. Hu, and G. Li, J. Chromatogr. A 1316, 29–36 (2013).
(13) Z. Shi, Y. Chen, L. Xia, G. Li, and Y. Hu, Sensor Actuat B-Chem. 350, 130908 (2022).
(14) Z. Chen, G. Li, and Z. Zhang, Anal. Chim. Acta 1065, 29–39 (2019).
(15) Y. Du, L. Xia, X. Xiao, G. Li, and X. Chen, J. Chromatogr. A 1554, 37–44 (2018).
(16) Y. Zhong, Y. Chen, Y. Hu, G. Li, and X. Xiao, Anal. Chem. 93, 16203–16212 (2021).
(17) R. Zhang, Y. Zhong, Z. Lu, Y. Chen, and G. Li, Chem. Sci. 12, 660–668 (2021).
(18) H. Zhang, H. Lai, X. Wu, G. Li, and Y. Hu, Anal. Chem. 92, 4607–4613 (2020).
(19) C. Zhang, G. Li, and Z. Zhang, J. Chromatogr. A 1419, 1–9 (2015).
Xia Ling and Gongke Li are with the School of Chemistry at Sun Yat-sen University, in Guangzhou, China. Direct correspondence to: cesgkl@mail.sysu.edu.cn


Thermodynamic Insights into Organic Solvent Extraction for Chemical Analysis of Medical Devices
April 16th 2025A new study, published by a researcher from Chemical Characterization Solutions in Minnesota, explored a new approach for sample preparation for the chemical characterization of medical devices.
Study Explores Thin-Film Extraction of Biogenic Amines via HPLC-MS/MS
March 27th 2025Scientists from Tabriz University and the University of Tabriz explored cellulose acetate-UiO-66-COOH as an affordable coating sorbent for thin film extraction of biogenic amines from cheese and alcohol-free beverages using HPLC-MS/MS.
Multi-Step Preparative LC–MS Workflow for Peptide Purification
March 21st 2025This article introduces a multi-step preparative purification workflow for synthetic peptides using liquid chromatography–mass spectrometry (LC–MS). The process involves optimizing separation conditions, scaling-up, fractionating, and confirming purity and recovery, using a single LC–MS system. High purity and recovery rates for synthetic peptides such as parathormone (PTH) are achieved. The method allows efficient purification and accurate confirmation of peptide synthesis and is suitable for handling complex preparative purification tasks.