Overcoming Common Challenges to Determine Residual Impurities Using IC in APIs with Limited Water
Organic solvents are generally not compatible with ion chromatography (IC) systems. The presence of organic solvents in sample diluent when injected onto an ion chromatographic system can cause unwanted chromatography artifacts such as broad baseline interferences or drifting baseline, which can preclude method robustness and accuracy. However, some active pharmaceutical ingredients (APIs) have poor water solubility, leaving the analyst with little choice other than to use organic solvents to attain dissolution. The approach presented here assists the use of organic solvents for sample preparation and provides a mechanism for the removal of the organic solvents from the chromatographic flow path. This removes all associated chromatographic interferences, thereby providing a good basis for an accurate and robust method.
A common active pharmaceutical ingredient (API) specification quality attribute is to test for ionic impurities at low levels. The technique of choice for testing this quality attribute would typically be ion chromatography with conductivity detection (IC–CD). Conductivity detection operates best with an aqueous-based mobile phase. The challenge for the analyst is that they often have little choice other than to prepare the sample solution in an organic solvent, meaning they are then faced with a compromised method performance.
Based on significant personal experience using ion chromatography techniques, it has been observed that the presence of organic solvents in the analytical flow path of ion chromatographic systems can cause unwanted artifacts on the conductivity detector baseline.
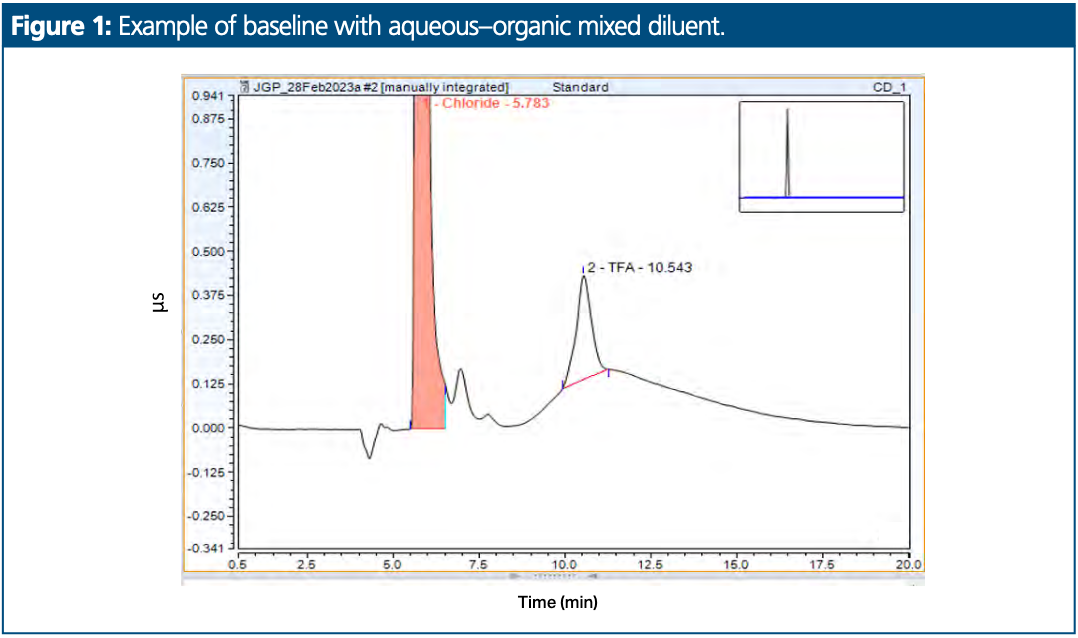
The two analytical method case studies described here (chloride and trifluoroacetic acid determination and residual propane phosphonic acid anhydride determination) were subject to analytical inaccuracy because the presence of the sample acetonitrile diluent in the analytical flow path elicited a significant baseline disturbance. This disturbance adversely impacted method accuracy by preventing consistent peak integration (Figure 1). This article describes an experimental setup where the organic solvent used in the sample preparation can be eliminated from the analytical flow path of the ion chromatographic system to prevent unwanted conductivity detector artifacts.
A Solution to Remove the IC Baseline Disturbances
The technique presented enables sample preparation for ion chromatography to be completed using a diluent containing an organic solvent, which will not compromise the end chromatographic result. The key component of this experimental setup is a concentrator column.
The concentrator columns used were designed primarily for high-purity water analysis. The concentrator column retains anions from a measured volume of aqueous sample matrix, concentrating the analyte species and lowering detection limits by 2–5 orders of magnitude.
In these case studies, the concentrator column was used for the baseline improvement. The sample injection was performed in a three-step process:
- Load the sample on the sample loop as per a conventional system.
- Inject the sample solution on to the column concentrator and fix the analyte anions. Eliminate the organic diluent and hydrophobic components of the sample.
- Remove the analyte anions from the column concentrator and inject it on to the column for analysis.
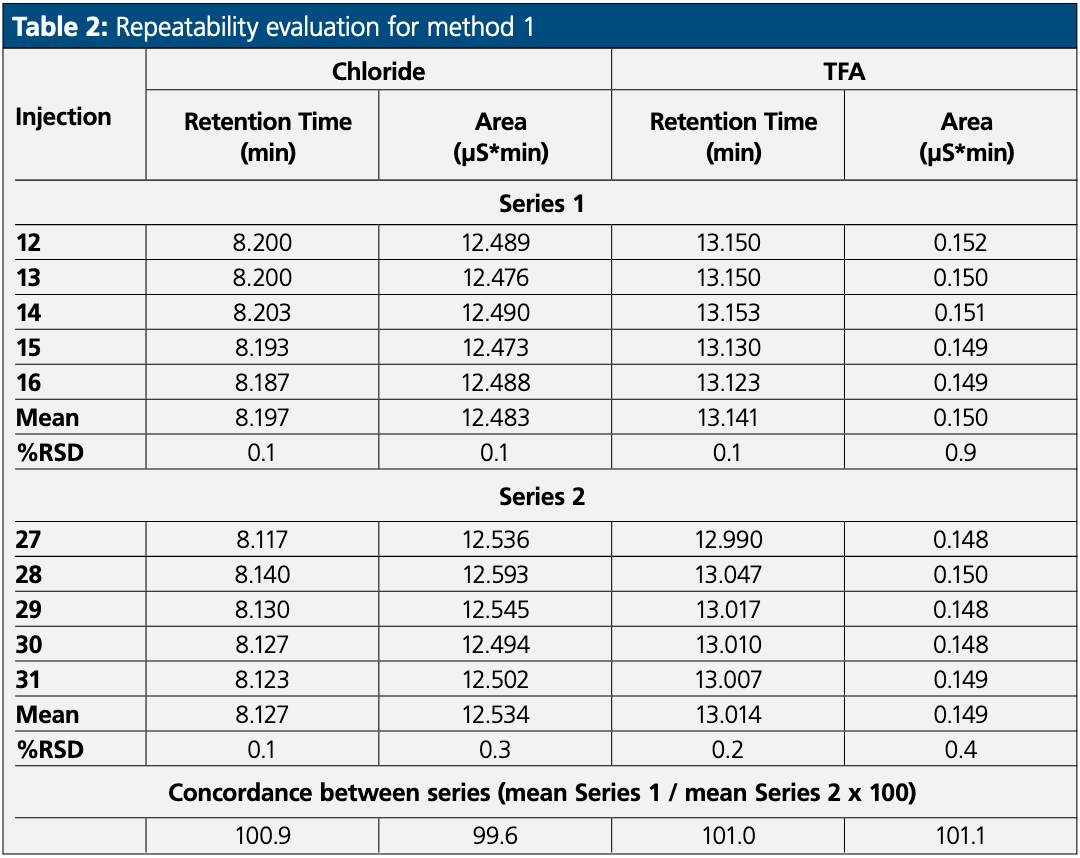
The repeatability of the retention time and the peak response was evaluated to assess if the column concentrator was suitable for a counterion method.
Principle of Operation of Conductivity Detectors
Conductivity detectors are designed to continuously monitor the electrical conductivity of water passing through a detector cell. When an analyte passes through the detector cell, there is a difference in the background conductivity of the water-based eluent and the change in this conductance when an analyte passes through the detector cell. The conductivity cell operates optimally for water-based eluents. If organic solvents are present in the diluent, then this is typically exhibited as a baseline disturbance as the organic solvent passes through the conductivity cell. This baseline disturbance is often very significant and, in some cases, can be of a comparable size to the analyte peaks, which can preclude consistent and accurate integration and compromise the method sensitivity. Figure 1 provides an example of such a baseline disturbance.
Solubility of Test Substances
Test substances in the form of a salt are typically amenable to analysis by IC because they readily dissolve in aqueous media. Other substances may not be completely soluble in aqueous solutions and there may be a necessity to introduce an organic solvent into the sample preparation diluent. The presence of the organic solvents in the diluent can then cause the described baseline disturbances.
Experimental
The ion chromatography system was set up as per the method testing requirements (see Table 1 for all manufacturer details).
Instrumental Setup
The anion trap column was installed on system 1 for the UHQ (ultra high quality) water source and the column concentrator fitted to system 2. The injection valve of system 1 was controlled by the autosampler (AS) and the injection valve of system 2 controlled by the detector (DC).
Sample Loading
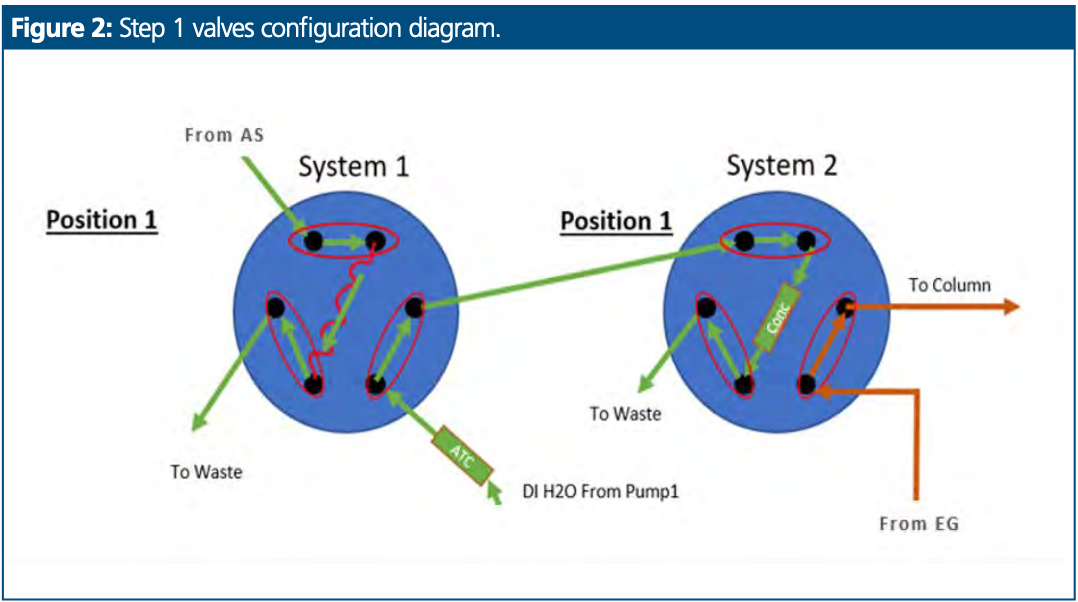
First Step: Sample Loading on Sample Loop
Sample was loaded into the sample loop in system 1 from the autosampler as for a conventional system and valve 2 was configured to equilibrate the column with eluent from the eluent generator (EG), as presented in Figure 2.
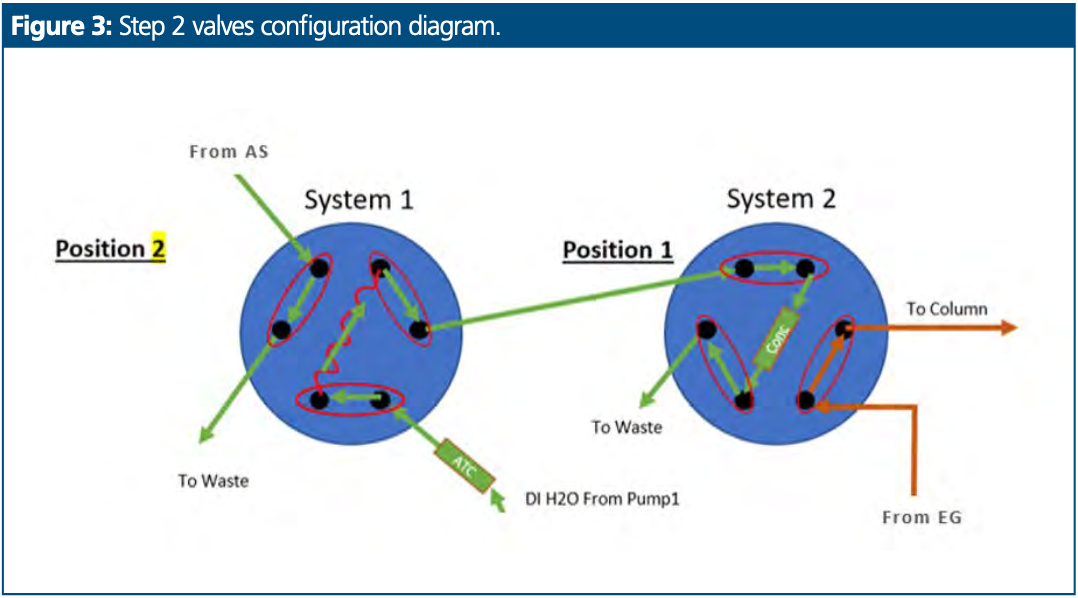
Second Step: Sample Loading on Concentrator Column and Diluent Elimination
Sample was rinsed from the sample loop in system 1 to be loaded on to the concentrator column in system 2. The matrix elimination process was then started, and the concentrator trapped the trace anions while pump 1 rinsed the aqueous–organic solvent to waste, as presented in Figure 3. This ran for a duration of 2 min.
Third Step: Sample Removed from Concentrator Column for Analysis
After 2 min, the matrix elimination ended and the trace anions were backflushed and removed from the concentrator column to be injected onto the analytical system, as presented in Figure 4.

Applications
Chloride and Trifluoroacetic Acid (TFA) Content Determination
A method for chloride and trifluoroacetic acid (TFA) content determination was selected first for evaluation. This was selected because the test sample material was not water soluble and was prepared in a mixture of 50:50 (v/v) water–acetonitrile. Figure 5 presents two overlaid chromatograms. The blue overlay represents a chromatogram where a sample prepared in 50:50 (v/v) water–acetonitrile was injected onto the column using a standard system setup. An interfering baseline hump is present at the retention time of TFA, which prevents suitable and consistent integration. The black overlay represents a chromatogram where a sample prepared in 50:50 (v/v) water–acetonitrile was injected onto the column using the system setup with the anion concentrator column. No baseline hump is apparent.
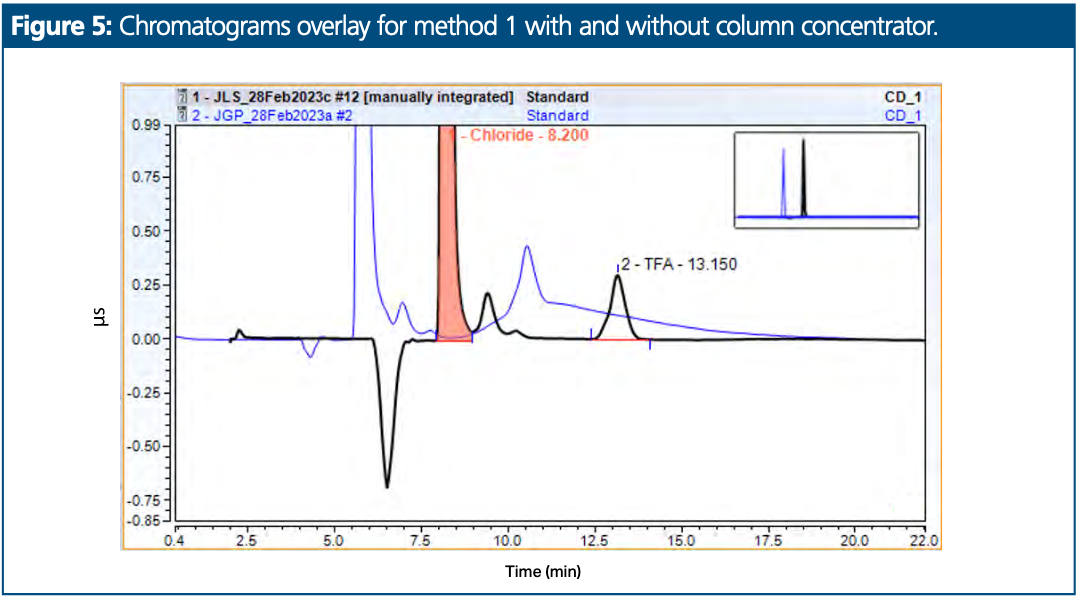
The repeatability evaluation was performed on five injections and repeated a second time after 10 injections of diluent. The results are presented in Table 2.
The chromatogram starting point with column concentrator was delayed by 2 min. The chromatogram starting point delay is to allow time for the analytes to be flushed onto the concentrator column during step 2 of the injection process.
Residual Propane Phosphonic Acid Anhydride
A second method, this time for residual propane phosphonic acid anhydride (Figure 6) content determination in an API, was tested. The sample was not soluble in water and was prepared in a mix of 50:50 (v/v) water–acetonitrile. Chromatograms overlaid with and without concentrator column are presented in Figure 7. The black trace represents a chromatogram obtained with the standard method setup. The blue trace represents a chromatogram obtained with the anionic concentrator column setup.
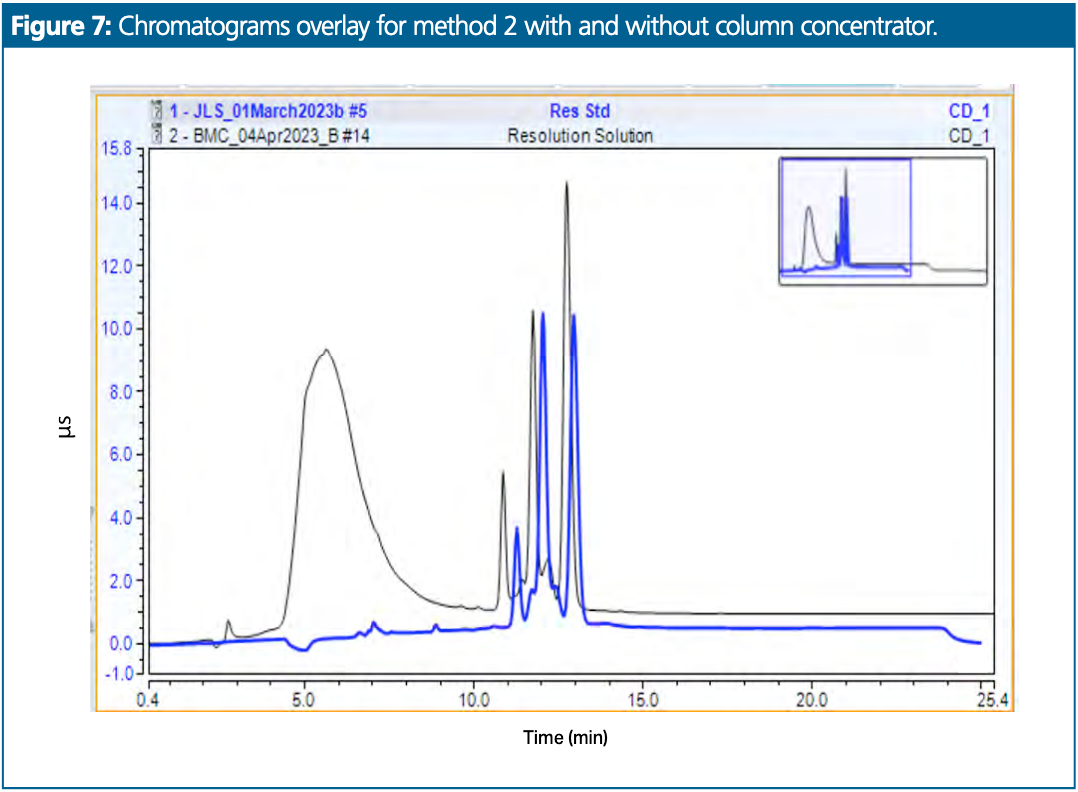
For the setup with the concentrator column, repeatability was not fully evaluated, but, as can be seen in Figure 8, three consecutive injection chromatograms are perfectly overlaid.

The baseline hump originating from the acetonitrile was completely removed, making the baseline more amenable for accurate and consistent integration. Differences in the overlays of triplicate standard injections were indistinguishable, indicating that the precision was very high.
Conclusions
The setup presented here provides the analyst with a solution to this challenge by facilitating the use of an organic solvent to solve the problem of effective sample preparation dissolution that does not then impact the method performance through a compromised detector baseline quality. The hardware setup would be a worthwhile consideration for future similar method development scenarios. A summary of the advantages of this approach are listed below.
Easy Setup
The setup does not require significant capital expenditure; the only additional items required are the additional tubing and the anionic concentrator column. Cationic variants of the concentrator columns are available for cationic chromatography methods.
Minor Changes to the Standard Setup Instrument Method
The changes required are minimal. The changes consist of modifications of the instrument control and the addition of an extra 2 min to the method run time to facilitate loading of the concentrator column.
Removed Interference from Organic Diluents
Baseline artifacts originating from the presence of organic diluent are eliminated, offering more consistent and accurate chromatogram integration.
Excellent Repeatability
The hardware setup with the concentrator yields very high injection precision.
Acknowledgments
- Reg Godwin, Applications Scientists at Thermo Fisher
- Jerome Pretre, Analytical Technical Leader at Almac Sciences
About the Author
James Sweatman holds a bachelor’s degree in chemistry from the University of Liverpool, UK, and a master’s degree in analytical chemistry from the University of Huddersfield, UK, and is currently an analytical investigator at Almac Sciences in Northern Ireland. With a career in analytical chemistry spanning over 28 years in the environmental and healthcare fields, he is an expert in analytical method development for small molecules with various chromatographic and spectroscopic techniques. Since joining Almac Sciences in 2007, he has led the analytical development and validation support for many small molecule GMP manufacturing campaigns. Direct correspondence to: james.sweatman@almacgroup.com

New Method Explored for the Detection of CECs in Crops Irrigated with Contaminated Water
April 30th 2025This new study presents a validated QuEChERS–LC-MS/MS method for detecting eight persistent, mobile, and toxic substances in escarole, tomatoes, and tomato leaves irrigated with contaminated water.
University of Tasmania Researchers Explore Haloacetic Acid Determiniation in Water with capLC–MS
April 29th 2025Haloacetic acid detection has become important when analyzing drinking and swimming pool water. University of Tasmania researchers have begun applying capillary liquid chromatography as a means of detecting these substances.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)















