Valves for Gas Chromatography, Part II: Applications
LCGC North America
Discussion about the use of gas switching valves for column backflush and two-dimensional series-bypass applications
In the second installment of this series, John Hinshaw discusses the use of gas switching valves for column backflush and two-dimensional series-bypass applications.
Multidimensional gas chromatography (MDGC) as practiced today may be divided into two main application areas: Classical MDGC and comprehensive two-dimensional gas chromatography (GC×GC).
Classical MDGC involves redirecting a portion, or portions, of the eluent from one column to another column with different separation characteristics, at times that are synchronized with the elution of specific peaks (or groups of peaks) from the first column.
The more recent technique of GC×GC also involves the transfer of the eluent from one column to another, but the transfer occurs cyclically and continuously across the entire chromatogram without targeting the timing of individual peaks. All of the eluent from the first column is transferred to the second column so that all peaks are separated by both columns. The comprehensive approach yields resolving powers that approach the product of the first and second columns with a corresponding leap in data quantity and complexity. Increased knowledge and skills are required from analysts to use this approach effectively in the routine laboratory.
This month's "GC Connections" will describe some basic examples of classical MDGC and offers insights into the more advanced methods of GC×GC.
Valve Applications
The injection of gaseous samples from a fixed-volume loop or the swapping of detectors during a GC analysis are two simple examples of ways that chromatographers use gas switching valves (GSVs).
The previous installment (1) showed how these fundamental valve techniques work for packed and micropacked GC columns. Chromatographers also use valves in more-complex ways, such as reversing the flow of carrier gas through a column, or to manipulate and redirect gas streams between two or more columns in multidimensional GC.
MDGC techniques enable the separation of a wide range of components that might not be achievable with a single column or in a single analysis. Separating a wide range of compounds with conventional single-column GC may require column temperature programming across a relatively large temperature range, and MDGC could provide an equivalent separation isothermally, sometimes in combination with carrier gas pressure programming.
Dual-parallel-column analyses are possible, too, but sometimes it is not desirable to inject the entire sample onto both columns, especially if all injected substances cannot be cleared from both columns before a subsequent injection.
Performing a pair of discrete separations one after the other is also possible, but this method would also place the entire sample into each column, as well as taking the sum of both analysis times to be completed.
Before looking at multidimensional applications, we'll first examine a simple column flow-reversal application.
Backflushing
Chromatographers use column backflushing when the sample contains residual materials that would not otherwise be eluted from a column until well after all peaks of interest have been eluted; as an alternative to high-temperature column oven excursions where the upper temperature limit may be curtailed by the maximum temperatures allowed for the valves or column; or if temperature cycling is not otherwise required. Column and valve lifetimes can be extended by avoiding repeated high temperatures over long time periods. Backflushing works by removing late-eluted impurities from a column that might otherwise interfere with subsequent analyses.
Backflushing of a single column can be accomplished using the six-port valve arrangement shown in Figure 1. In Figure 1a the six-port valve starts in the backflush or standby position. Carrier gas flow proceeds through the column from left to right as shown. The set-up is ready for injection after the sample fills the sample loop.
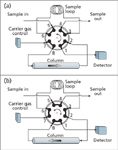
Figure 1: Single-column backflush to detector configuration. (a) Standbyâbackflush position, (b) Injectionâelution position.
When the valve is moved to the position shown in Figure 1b, the sample loop is flushed by carrier gas flow onto the right-hand column connection while the column flow direction reverses and starts going from right to left. As long as the sample loop pressure is low, there is no danger of the sample flowing backward into the carrier gas pneumatics because the carrier gas pressure is higher than the low-pressure end of the column at valve port 2 at the moment of injection.
The valve remains in the injection–elution position (Figure 1b) until the last peak of interest has been eluted. The valve is then returned to the standby–backflush position (Figure 1a), which causes the column flow to be restored to its original left-to-right direction (backward in relation to the direction that the carrier gas flowed during peak elution). Any solutes or impurities left in the column at the backflush time will begin to move in reverse, toward the detector. As the total run time approaches twice the backflush time — assuming the carrier gas flow or pressure are not modified — residual material will be backflushed through the detector. At a time equal to approximately twice the backflush time nearly all of the material will have been flushed out. Some may remain as a result of band-broadening effects or nonlinear retention, so it is good practice not to perform another injection until sufficient additional time has passed in the backflush mode to remove all residual material.
Figure 2a illustrates a backflushed chromatogram of a simple gas mixture. In Figure 2b, the backflushed portion of the chromatogram is enlarged 200× to show a momentary baseline disturbance at the backflush time of 480 s, followed by some unidentified C3 and heavier compounds and a return to baseline before 960 s, at twice the backflushing time. Although the impurity levels are relatively low in this example, if they are not purged from the column after each run such extraneous noneluted material could build up over many runs and cause baseline instability. Other examples include natural gas analysis with grouping of C6+ compounds, environmental extracts with high-boiling matrix compounds, and food and beverage volatiles.
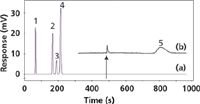
Figure 2: Column backflushing example. Conditions: 3 m à 1.0 mm porous polymer micropacked column; 70 °C isothermal; constant pressure; 1.0 mL injection; flame-ionization detection (FID). (a) scaled to peaks; (b) enlarged 200à Backflush time: 480 s, indicated by arrow. Peaks: 1 = methane, 2 = ethylene, 3 = acetylene, 4 = ethane, 5 = backflushed impurities.
The example shown here was performed on a micropacked column. The principles of backflushing for open-tubular columns are similar, but because of the low dead-volume requirements such separations may be better managed with various fluidic capillary switching devices. A future installment of "GC Connections" will address these configurations and their applications.
Column Sequence Reversal
Some columns yield good separations for certain compounds but not for others. For example, a molecular sieve column discriminates between various light gases such as hydrogen, oxygen, nitrogen, and carbon monoxide, but fails to separate water or carbon dioxide.
These two compounds will contaminate a molecular sieve column until baked off at high temperatures, and if they are allowed to accumulate they will degrade the separation of other compounds on the molecular sieve column. On the other hand, certain porous polymer columns easily separate water and carbon dioxide as well as a variety of hydrocarbons and other gases, but may not produce acceptable separations of those gases handled well by molecular sieves.
It is possible to combine the separation capabilities of these two columns for a complete separation of fixed gases and C1–C3 hydrocarbons. The rest of this installment will address the separation of these solutes in a couple of different ways. Other examples for different sets of solutes are easy to find in the chromatography literature.
Two popular methods for this type of combined column two-dimensional separation are sequence-reversal and series-bypass operation. In both cases, the flow and connections between two columns are manipulated during the analysis via valve operations so that each column receives and separates the peaks that it is best suited for.
The main differences between sequence reversal and series-bypass operations is that the former uses a single detector or two detectors in series, while the latter can use two discrete detectors for true dual simultaneous separations. Column sequence reversal is illustrated by the flow diagram in Figure 3; this configuration uses a 10-port valve. The details are not shown here for reasons of space, but several examples can be found on the manufacturers' websites.
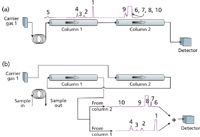
Figure 3: Flow diagram for column sequence reversal. (a) Injectionâsequential flow. (b) Sequence reversal. See text for description of operation. Peaks: 1 = carbon dioxide, 2 = ethylene, 3 = acetylene, 4 = ethane, 5 = impurities including water, 6 = hydrogen, 7 = oxygen, 8 = nitrogen, 9 = methane, 10 = carbon monoxide. Note: the time scale for chromatograms increases from right to left. Chromatograms are shown with thermal conductivity detection to reveal the fixed gases.
In sequence reversal some care must be taken to ensure that peaks from one column are not coeluted with peaks from the other. Figure 3a illustrates the column flows in the first step of injection with sequential flow from column 1 to column 2.
The sample is injected from the sample loop into column 1. The initial, partially separated peaks from column 1 enter column 2 until those peaks destined to be separated on column 2 have been transferred. The valve is then moved to change to flow as shown in Figure 3b; the sequence of the columns is reversed.
Peaks remaining in column 1 continue to partition and move along the column until they are subsequently eluted and detected. Simultaneously, peaks from column 2 are eluted into column 1, pass through it with little to no additional retention, and enter the detector. Coelution of peaks in a complex mixture such as in this example may be difficult to avoid. In some applications it may be necessary to add a second valve to stop flow in column 2 and delay its peaks from entering column 1 until the existing peaks in column 1 have been eluted.
A related configuration keeps the second column flowing to the detector, but backflushes the first column to vent after the solutes to be separated on the second column have left the first column. An independent carrier gas source may be used for backflushing the first column. This configuration is sometimes called precolumn backflushing, and can also be found in manufacturers' literature. It is useful when only the peaks from the second column are to be analyzed, and the remaining peaks in the first column can be discarded.
Series-Bypass Backflush Operation
For the application at hand, what we really want to accomplish is the simultaneous determination of all of the peaks. This can be made possible by combining the two applications discussed so far, backflushing and column-to-column flow redirection. The backflush application shown in Figure 1 is augmented by replacing the six-port valve with a 10-port valve, and an additional six-port valve, carrier gas source, and detector are added. The 10-port valve provides the backflush functionality, and the six-port valve controls the destination of the effluent from column 1 — either to column 2 or to detector 1 through a restrictor — while generating functionality similar to that of the sequence-reversal configuration of Figure 3.
Figure 4 illustrates the flow paths of a series-bypass backflush configuration. In Figure 4a, residual material from a previous injection is backflushed from column 1 to detector 1. The configuration remains in this position until the sample loop is filled and injection may be started. Figure 4b shows the flows for injection and series operation of column 1 into column 2. Here, the contents of the sample loop are injected into column 1 and the initial unresolved fixed-gas peaks plus methane are passed in series onto column 2. After the methane peak passes into column 2, the valves are repositioned to produce the flow paths shown in Figure 4c.
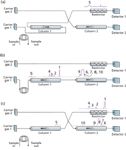
Figure 4: Flow diagram for series-bypass operation with precolumn backflush. Discrete detectors for each column channel eliminate peak overlaps. (a) Standbyâbackflush flow, (b) Injectâseries flow, and (c) bypass flow. Peaks as labeled in Figure 3. Note: the time scale for chromatograms increases from right to left. Chromatograms are shown with thermal conductivity detection to reveal the fixed gases.
Column 1 now bypasses column 2, and both columns flow to their respective detectors. The solutes that were initially passed from column 1 to column 2 are eluted into detector 2, while the remaining target peaks from column 1 are eluted into detector 1. After the column 1 target peaks have been eluted, the flows are reset to the position shown in Figure 4a, so that any remaining contaminants in column 1 are backflushed to detector 1. The restrictor, which has similar flow characteristics to column 2, serves to maintain a more constant flow in column 1 as it is switched in front of column 2 and back again.
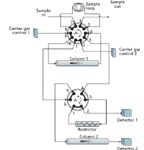
Figure 5: Series-bypass with backflush valve diagram. Both valves shown in the A position, for standbyâbackflush operation. See Table I for the relationship of valve positions to the flow paths shown in Figure 4.
The valve plumbing for the series-bypass backflush configuration is shown in Figure 5. The 10-port valve provides sample injection and backflush functionality, and the six-port valve selects whether column 1 effluent is directed to column 2 or to detector 1 via the restrictor. The valve positions in Figure 5 that correlate with the three flow configurations of Figure 4 are listed in Table I.
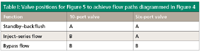
Table I: Valve positions for Figure 5 to achieve flow paths diagrammed in Figure 4
The timing of the bypass operation is important. If the first column is bypassed around the second column too soon, then some of the peaks that should enter the second column do not. If the bypass time is set too long, then some of the peaks that should remain in the first column can enter the second column instead. Figure 6 shows the effects of increasing the bypass time by 20 s. The methane peak is split between the first and second columns with a 60-s bypass time. Increasing the bypass time by 20 s results in all of the methane appearing on the second column. This timing must be revalidated any time the flow, temperatures, or the columns themselves are changed. The timing of the backflush operation, at 480 s in this example, is not as critical and can occur at any time that the transient spike does not interfere with a peak, after all of the peaks of interest have been eluted from column 1.
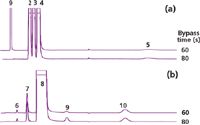
Figure 6: Tuning the bypass time. (a) Porous polymer column with flame-ionization detection (column 1 in Figures 4 and 5); (b) molecular sieve 5A column with thermal-conductivity detection (column 2 in Figures 4 and 5). The methane peak (9) is split between the two columns at a bypass time of 60 s and is passed entirely to the molecular sieve column at a bypass time of 80 s. Peak labels as in Figure 3.
Conclusion
This month's installment examined the basics of valve applications for backflushing, sequence reversal, and pre-column bypassing. We then combined these basic techniques to create a hybrid dual-valve configuration capable of separating both fixed gases and hydrocarbons in a single two-dimensional analysis. MDGC presents a powerful suite of techniques that analytical chemists can use to tease apart solutes that might otherwise be inseparable, at least without significant additional effort. The next installment in this series will discuss multidimensional separations as applied to open-tubular columns; including fluidic flow switching and applications to heart-cutting, selectivity tuning of serially connected columns and GC×GC separations.
References
(1) J. Hinshaw, LCGC North America 29(3), 246–251 (2011).
John V. Hinshaw "GC Connections" editor John V. Hinshaw is Senior Scientist at BPL Global, Ltd., Hillsboro, Oregon, and a member of LCGC's editorial advisory board. Direct correspondence about this column to the author via e-mail: lcgcedit@lcgcmag.com.

John V. Hinshaw

New Study Reviews Chromatography Methods for Flavonoid Analysis
April 21st 2025Flavonoids are widely used metabolites that carry out various functions in different industries, such as food and cosmetics. Detecting, separating, and quantifying them in fruit species can be a complicated process.
Quantifying Terpenes in Hydrodistilled Cannabis sativa Essential Oil with GC-MS
April 21st 2025A recent study conducted at the University of Georgia, (Athens, Georgia) presented a validated method for quantifying 18 terpenes in Cannabis sativa essential oil, extracted via hydrodistillation. The method, utilizing gas chromatography–mass spectrometry (GC–MS) with selected ion monitoring (SIM), includes using internal standards (n-tridecane and octadecane) for accurate analysis, with key validation parameters—such as specificity, accuracy, precision, and detection limits—thoroughly assessed. LCGC International spoke to Noelle Joy of the University of Georgia, corresponding author of this paper discussing the method, about its creation and benefits it offers the analytical community.



















