Comprehensive Analysis of Persistent Organic Pollutants in Complex Matrices Using GC with High-Performance TOF-MS
The combination of GC with time-of-flight (TOF)-MS proves to be a successful approach for the challenging analysis of persistent organic pollutants in complex matrices such as sediment and fish samples.
The analysis of persistent organic pollutants (POPs) in complex matrices is challenging because of their broad concentration ranges and the presence of interfering substances with similar physicochemical properties. In addition, there is a lack of instrument technology for widespread analyses of targeted and nontargeted compounds in minimally processed environmental samples. POPs in complex matrices such as sediment and fish samples were analyzed using gas chromatography (GC) with high-performance time-of-flight mass spectrometry (TOF-MS). High-performance TOF-MS offers the benefits of classical TOF (for example, a nonscanning mass analyzer, high acquisition rates, and spectral continuity) with the added advantages of increased mass accuracy and resolving power (R = 50,000 at FWHM in ultrahigh resolution mode). Different classes of POPs were screened in single sample analyses. The power of high-resolution MS coupled with the ability to extract specific ions from full-range mass spectral data makes high-performance TOF-MS valuable for analyses of POPs in complex matrices.
Persistent organic pollutants (POPs) are halogenated organic compounds produced industrially for a wide variety of commercial applications. Examples of POPs include dioxins (polychlorinated dibenzodioxins [PCDDs] and polychlorinated dibenzofurans [PCDFs]), polychlorinated biphenyls (PCBs), and polybrominated diphenyl ethers (PBDEs) (Figure 1). PCBs, marketed under trade names such as Aroclor, Sovol, and Clophen, were used for many years as dielectric fluids in electrical transformers and capacitors, heat transfer fluids, hydraulic fluids, lubricating and cutting oils, and additives in many commercial products (1). PBDEs have been used as fire retardants in household goods, construction material, and various plastics for the aviation and automotive industry (2). Dioxins are by-products formed during combustion processes. POPs are relatively inert and lipophilic, and tend to bioaccumulate in the environment. Their adverse health and environmental effects have led to their regulation in many industrialized countries (3,4). Detection of POPs is complicated by their broad range of concentrations and the presence of interfering substances with similar physicochemical properties (5–7). Furthermore, there has been a lack of appropriate instrumental technology for exhaustive analyses of targeted and nontargeted POPs in minimally processed environmental samples. Analysis of environmental samples typically requires costly equipment and highly specialized personnel. Analysts can spend significant time isolating, purifying, and characterizing the materials only to find that their efforts have resulted in partial loss, chemical modification, or complete elimination of targeted substances.
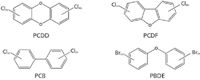
Figure 1: General structures for POPs.
Comprehensive analysis of POPs should involve the study of all classes of POPs and their metabolites. Metabolites of POPs are emerging as regular contaminants in wildlife and are critical for the risk assessment of human exposure to pollutants such as PCBs (8). Detection of POPs can be improved by reducing the amount of sample processing or enhancing instrument selectivity. High-resolution mass spectrometry (MS) has been the method of choice for the analysis of POPs; however, time-of-flight (TOF) mass spectrometers have emerged as an attractive alternative to their more costly counterparts (9,10). TOF-MS instruments were commercially available in the 1950s, and recent advances such as orthogonal acceleration, extended pathlengths, and improvements in data acquisition and processing have led to a renaissance of TOF mass analyzers as modern tools for biological and chemical research (9). The attractive features of TOF-MS, such as an unlimited mass range, fast acquisition rates, and spectral acquisition over a wide mass range without sacrifice of sensitivity, have been advantageous for the analysis of both small and large molecules. The fast acquisition rates of TOF-MS result in nonskewed mass spectral data that can be easily deconvoluted. Unfortunately, the low-resolution capabilities of classical TOF instruments made them unsuitable for the analysis of POPs. The emergence of high performance TOF-MS with extended flight paths, improved detection systems, and resolving powers greater than 50,000 (FWHM), has only increased the utility of these instruments for both targeted and nontargeted analysis of complex samples (7,11).
Experimental
Gas chromatography (GC)–TOF-MS analyses of fish and sediment samples containing PCBs and other POPs were performed using a Pegasus GC-HRT MS system (LECO Corporation, St. Joseph, Michigan) equipped with an Agilent Technologies (Palo Alto, California) 7890A GC system. PCB-containing sediment and fish samples were analyzed using an Rxi-5 Sil MS column (Restek, Bellefonte, Pennsylvania) and data were collected in high-resolution mode. Samples were previously prepared and not processed further before analysis (12). GC and MS parameters for PCB sample analyses are listed in Table I.
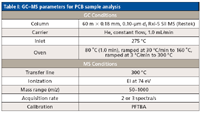
Table I: GCâMS parameters for PCB sample analysis
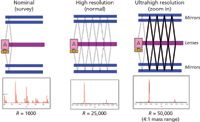
Figure 2: TOF-MS system operational modes (Pegasus GC-HRT). A = accelerator, D = detector.
At the heart of the MS system is a folded flight path (FFP) mass analyzer with three operational modes (Figure 2): nominal (resolving power, R = 1000, FWHM at 219 m/z), high resolution (R = 25,000, FWHM at 218.985080), and ultrahigh resolution (R = 50,000, FWHM at 218.985080). Ions are introduced via orthogonal acceleration and then travel between a parallel set of gridless mirrors and through a set of periodic ion lenses (Figure 3). The flight path can be adjusted to 2 m (nominal), 20 m (high resolution), or 40 m (ultrahigh resolution). Most routine analyses can be performed in high-resolution mode; however, nominal mode can be used for quick inspection of samples, chromatographic method development, or NIST library searches. Ultrahigh-resolution mode can be used for samples requiring a high level of selectivity.
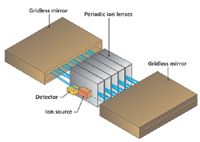
Figure 3: FFP mass analyzer.

Figure 4: (a) TIC of sediment containing elevated PCB levels. (b) XIC of sediment sample showing three different PCBs.
Results and Discussion
The utility of high-performance TOF-MS is clearly evident from the analysis of sediment sample containing elevated levels of PCBs (>90 congeners). The total ion chromatogram (TIC) and extracted ion chromatogram (XIC) are shown in Figure 4. The average mass accuracy for 26 PCBs present in the sediment is -0.71 ppm with a standard deviation of 0.81 (Table II). Mass spectral data for PCBs 47, 162, and 209 are shown in Figure 5.
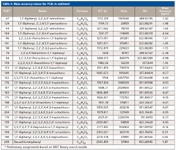
Table II: Mass accuracy values for PCBs in sediment

Figure 5: Mass spectra for PCBs (a) 47, (b) 162, and (c) 209.
Comprehensive mass spectral analysis of trace levels of PCBs and other POPs was accomplished using high-performance TOF-MS. The analysis of fish tissue resulted in the identification of 15 PCBs (for example, tri-, penta-, hexa-, hepta, octa-, nona-, and deca-PCB congeners) and other classes of POPs such as dichlorodiphenyldichloroethene (p,p'-DDE). p,p'-DDE is the major metabolite of dichlorodiphenyltrichloroethane (DDT). p,p'-DDE has been linked to cancer and reproductive deficiencies in humans (13,14). TIC and XIC data (p,p'-DDE, several hexachloro-PCBs including PCB 153 and decachlorobiphenyl) for fish tissue are shown in Figure 6. The molecular ion region in the corresponding mass spectral data is shown in Figure 7. A comparison of the calculated and observed ion ratios in the molecular ion region of the data is shown in Table III. The TOF-MS system with its high resolving power provided good mass accuracies for the three analytes: –0.10 ppm (p,p'-DDE), 0.79 ppm (PCB 153), and –0.08 (PCB 209).
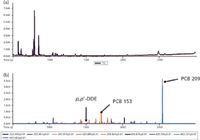
Figure 6: (a) TIC of fish samples containing low levels of PCBs. (b) XIC of sediment sample showing two different PCBs and p,p’-DDE.
Sector mass analyzers have traditionally been used for the analysis of POPs. However, TOF-MS systems are the ideal detectors for comprehensive POP analysis. Their fast acquisition rates, broad mass spectral range, and inherent sensitivity have made them an attractive option over conventional scanning mass spectrometers (12). With TOF-MS a complete data set can be acquired in a single run. Selection of desired analytes is performed after the acquisition using accompanying software that allows users to extract specific ions from the total ion set. This is in contrast to sector instruments that are operated in multiple ion detection (MID) mode to perform quantitative high-resolution spectrometry and quadrupole analyzers that are scanned across a given mass range or more typically set to acquire in selective ion monitoring (SIM) mode. The ability to simultaneously extract ions for different classes of POPs and possible metabolites would clearly facilitate analysis of complex environmental samples. One problem that has plagued TOF-MS instruments in the past is their relatively low resolving power caused by varying kinetic energy distributions in the ion source and mass analyzer. These energy or time aberrations have been reduced through the use of orthogonal acceleration, delayed extraction, and reflectrons (10). The high-performance mass analyzer minimizes these time aberrations and provides a flight path of up to 40 m, resulting in high mass accuracy and resolving power.
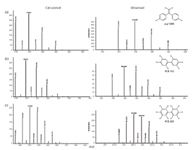
Figure 7: Calculated and observed molecular ion region of the mass spectrum: (a) p,p’-DDE, (b) PCB 153, and (c) PCB 209.
Conclusion
Analysis of POPs is challenging due to the similar chemical and physical properties of these compounds. They are aromatic, lipophilic, and usually coextracted with numerous organic compounds when nonpolar solvents (for example, hexanes) are used during sample preparation. High-performance TOF-MS is capable of performing exact mass measurements and allows for selection and extraction of targeted analytes for elemental composition confirmation. High-performance TOF-MS provides a comprehensive profile of the sample in a single run, making it a powerful tool for the analysis of POPs in complex matrices.

Table III: Isotope ratio values for p,p’-DDE, PCB 153, and PCB 209
Acknowledgment
The authors would like to thank Dr. Eric Reiner from the Ontario Ministry of the Environment (Toronto, ON) for providing samples.
References
(1) S.K. Shin and T.S. Kim, J. Hazard Mater. 137(3), 1514–1522 (2006).
(2) M.A. Siddiqi, Clin.Med. & Res. 1(4), 281–290 (2003).
(3) P.O. Darnerud, G.S. Eriksen, T. Johannesson, P.B. Larsen, and M. Viluksela, Environ. Health Perspect. 1, 49–68 (2001).
(4) E.J. Reiner, A.R. Boden, T. Chen , K.A. MacPherson, and A.M. Muscalu, LCGC Eur. 23(2), 60 (2010).
(5) T.M. Kolic, L. Shen, K. MacPherson, L. Fayez, T. Gobran, P.A. Helm, C.H. Marvin, G. Arsenault, and E. Reiner, J Chrom. Sci. 47, 83–91 (2009).
(6) H.M. Stapleton, Anal. Bioanal. Chem. 386, 807–817 (2006).
(7) T. Cajka, J. Hajslova, R. Kazda, and J. Poustka, J. Sep. Sci. 28, 601–611 (2005).
(8) J. Maervoet, A. Covaci, P. Schepens, C.D. Sandau, and R.J. Letcher, Environ Health Perspect. 112(3), 291–294 (2004).
(9) A.W.T. Bristow, Mass Spectrom. Rev. 25, 99–111 (2006).
(10) E. de Hoffmann and V. Stroobant Mass Spectrometry — Principles and Applications (John Wiley & Sons, Hoboken, New Jersey, 3rd ed., 2007), pp. 126–142.
(11) J.F. Holland and B.D. Gardner in Flavor, Fragrance, and Odor Analysis, R. Marsili, Ed. (Marcel Dekker, Inc., 2002), pp. 107–138.
(12) T.M. Kolic, L. Shen, K. MacPherson, L. Fayez, T. Gobran, P.A. Helm, C.H. Marvin, G. Arsenault, and E.J. Reiner, J. Chrom. Sci. 47, 83–91 (2009).
(13) K. Jaga and D. Brosius, Rev. Environ. Health 14(1), 39–50 (1999).
(14) T. Tiido et al., Environ. Health Perspect. 114(5), 718–724 (2006).
Joe Binkley and Kevin Siek are with LECO Corporation Life Science & Chemical Analysis Center in St. Joseph, Michigan.
David E. Alonso is an applications chemist at LECO Corporation Life Science & Chemical Analysis Center in St. Joseph, Michigan.

Understanding FDA Recommendations for N-Nitrosamine Impurity Levels
April 17th 2025We spoke with Josh Hoerner, general manager of Purisys, which specializes in a small volume custom synthesis and specialized controlled substance manufacturing, to gain his perspective on FDA’s recommendations for acceptable intake limits for N-nitrosamine impurities.
University of Rouen-Normandy Scientists Explore Eco-Friendly Sampling Approach for GC-HRMS
April 17th 2025Root exudates—substances secreted by living plant roots—are challenging to sample, as they are typically extracted using artificial devices and can vary widely in both quantity and composition across plant species.
Determining the Serum Proteomic Profile in Migraine Patients with LC–MS
April 17th 2025Researchers used liquid chromatography–mass spectrometry (LC–MS) in their proteomic analysis to compare the serum proteome of migraine patients with healthy controls and to identify differentially expressed proteins as potential migraine biomarkers.



















