Determination of Clenbuterol-Like Beta-Agonist Residues in Hair
LCGC North America
LC–MS-MS is used to detect compounds relevant for food safety.
A liquid chromatography–tandem mass spectrometry (LC–MS-MS) method to determine clenbuterol-like beta-agonist residues in hair was developed and validated. Hair samples were extracted with 0.1 mol/L hydrochloric acid and 0.2 mol/L ammonium acetate buffer (pH 5.0) and cleaned up using a solid-phase extraction cartridge (60 mg/3 mL). Linearity was assessed in the 0.1–50 ng/L (r = 0.9951–0.9996) range for 18 beta-agonists in hair samples. The analytical recoveries were acceptable; between 72.76% and 122.22%, with intraday and interday precisions of relative standard deviation (RSD) between 1.6% and 17%, and the limits of detection were all lower than 0.5 ng/L. The proposed LC–MS-MS method has high sensitivity and strong specificity with a relatively easy procedure for sample preparation. Thus, it can be used to detect beta-agonist residues in hair, providing a basis for regulating food safety.
Beta-adrenoceptor agonists (β-agonists) are used to treat bronchiectasis and to increase pulmonary ventilation. Clenbuterol is the best known of the aniline-like β-agonists. When the drug dosage exceeds the treatment amount by 5–10-fold, it can cause a substantial increase in the muscle mass of animals such as cows, lambs, pigs, and poultry. Because the drug has stable chemical characteristics and cannot be destroyed by general heating, it remains in a significant residual amount in edible animal tissue. For example, clenbuterol is stable in 100 °C water and has a half life of 5 min in 260 °C oil (1). When a person eats the animal tissue (for example liver, kidney, lung, or meat), toxicity symptoms may develop (2). Thus, the public representatives of the European Economic Community in July 1997 approved a law to ban the use of β-agonists in all animals destined for human consumption (3). All β-agonists are currently banned from use in edible animals in China, the United States, and the European Union.
There have been few studies on the development of a standard method of assaying nondestructive living samples from animal hair. For this reason, we investigated the development of an assay using hair for the detection of β-agonists.
Materials and Methods
Reagents and Equipment
Methanol, ethyl acetate, acetonitrile, and formic acid were all high performance liquid chromatography (HPLC)–grade (Merck, Darmstadt, Germany). Sodium dihydrogen phosphate, hydrochloric acid, and ammonium hydroxide solution were of analytical-reagent grade. Water was produced by a Milli-Q system (Millipore Corp., Molsheim, France).
A clenbuterol hydrochloride reference substance was purchased from the National Institute for the Control of Pharmaceutical and Biological Products in China, which was equivalent to 88.38% clenbuterol. Terbutaline sulfate, salbutamol, fenoterol hydrobromide, and salmeterol were all in accordance with the European Pharmacopoeia; ractopamine hydrochloride, brombuterol hydrochloride, cimaterol, clenpenterol hydrochloride, mabuterol hydrochloride, tulobuterol hydrochloride, mapenterol hydrochloride, cimbuterol, and bambuterol hydrochloride were all supplied by WITEGA Laboratorien Berlin-Adlershof GmbH (Berlin, Germany); ritodrine hydrochloride, isoxsuprine hydrochloride, and penbutolol hydrochloride were all in accordance with the United States Pharmacopeia (USP); D9-clenbuterol and labetalol hydrochloride were all supplied by Sigma (Carlsbad, California).
Hair samples were obtained, both from pigs from the Shanghai hoggery and from people in the outskirts of Shanghai; the hair was processed, washed, and measured separately. Hair that tested drug-negative was regarded as a blank hair sample.
The equipment included an Agilent liquid chromatograph connected to a triple-quadrupole mass spectrometry (MS) detector with electrospray ionization (ESI) sources (Agilent Technologies Corp., Santa Clara, California); another Agilent liquid chromatography system, including a binary pump, degasser, column oven, and autosampler (Agilent Technologies, Waldbronn, Germany); an ultrasonic instrument (Shanghai Kudos Ultrasonic Instrument Corp., Shanghai, China); ultracentrifugation (Thermo Electron Corp., Osterode, Germany); a constant temperature incubator (Shanghai Medical Constant Temperature Equipment Factory, Shanghai, China); a vortex mixer (IKA Corp., Staufen, Germany); a nitrogen blower (Organomation Associates Inc., Berlin, Massachusetts); an electronic balance (Beijng Sartorius Balances Co., Beijing, China); a solid-phase extraction (SPE) manifold (Waters, Milford, Massachusetts); a PCX SPE column (60 mg/3 mL) (Tianjin Agela Technologies Corp., Tianjin, China); an MCX SPE column; and the Milli-Q water purification system (Millipore Corp., Molsheim, France).
Clenbuterol-Like β-Agonist Standard Solutions
Standard stock solutions of clenbuterol and clenbuterol-like β-agonists were prepared in methanol at a concentration of 100 μg/mL and stored at 4 °C. The standard stock solutions were used to prepare 100, 10, and 1 ng/mL standard solutions.
Internal Standard Solution
The internal standard (D9-clenbuterol isotope) was also prepared in methanol at a concentration of 100 μg/mL and stored at 4 °C. The internal standard stock solution was used to prepare a 500 ng/mL internal standard solution.
Pretreatment of Hair Samples
Wash
Each hair sample was washed once with 1% polysorbate-80 and three times with ultrapurified water, dried at 40 °C, cut into pieces, and stored in a dry place.
Extraction
A total of 500 mg of each washed hair sample was weighed, and 100 μL of internal standard solution (500 ng/mL) and 10 mL of 0.1 mol/L hydrochloric acid were added. This mixture was then incubated in a 40 °C water bath for 18 h, removed, cooled to room temperature, vibrated on a shaker for 10 min, and centrifuged at 4600 rpm for 5 min. The supernatant was removed and kept, and the residue dissolved in 5 mL of 0.2 M ammonium acetate buffer (pH 5.0). The vortex was mixed for 10 min, and centrifuged. The supernatants were combined.
Purification–Cleanup
A 60 mg/3 mL PCX column was activated sequentially with 3 mL of methanol, 3 mL of water, and 3 mL of 2% (w/v) formic acid. The sample supernatant was transferred to the top of the extraction column. The column was washed with 3 mL of 2% (w/v) formic acid solution and 3 mL of formic acid in turn, then dried for 15 min at a pressure of –10 mm Hg. Elution was conducted with 12 mL of 90:3 (v/v) saturated ammonium hydroxide–ethyl acetate. The eluate was collected and dried under nitrogen at 40 °C, reconstituted with 200 μL of 0.1% (w/v) formic acid, and then vortexed for 1 min for analysis.
Liquid Chromatography–Mass Spectrometry Analysis
HPLC Analysis
Chromatography was performed at 30 °C on a 100 mm × 3.0 mm, 1.8-μm dp HPLC Agilent C18 column at a flow rate of 0.4 mL/min, using 0.1% formic acid aqueous solution as mobile phase A and 0.1% formic acid in acetonitrile as mobile phase B. A total of 10 μL of each sample preparation was injected onto the HPLC column and eluted with a mobile phase gradient: 12%–70% B in 6.5 min and back to 12% B in 0.1 min, with the analytical column equilibrated in 4 min before the next injection. The liquid chromatograph was connected to the ESI MS-MS instrument.
MS Analysis
The mass spectrometer was operated in the positive ESI mode at a capillary voltage of 4.0 kV, with the ion source set at 350 °C, in a nitrogen gas flow of 11 L/min, nebulizer 35 psi. For each analyte, ion information was acquired under the conditions shown in Table I.
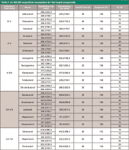
Table I: LCâMS-MS acquisition parameters for the target compounds
Results
Specificity
A 500-mg blank hair sample was taken. As described above, the chromatography results were recorded and compared with the spiked sample and internal standard. The total ion chromatograms (TICs) showed that the endogenous substances of the hair would not contaminate the test for 18 kinds of β-agonists in the hair (Figure 1).

Figure 1: (a) Blank hair TIC, (b) TIC of blank hair with internal standard added, and (c) TIC of blank hair with 18 β-agonists and internal standard added.
Linearity
A 500-mg blank hair sample was weighed and processed to give sample concentrations of 1, 2, 5, 10, 20, and 50 ng/g, following treatment and analysis as described earlier. Internal standard calibration curves demonstrated a linear relationship between the ratio integrated peak area and the internal quantitative ion, at a concentration range of 1.0–50.0 ng/g, correlative coefficient (γ) all above 0.99.
Precision
A 500-mg blank hair sample was weighed following the assay method evaluation and results as set out in the European Community guidelines (4). Hair samples at three standard concentrations (1.0, 1.5, and 2.0 ng/g) of clenbuterol-like β-agonist were prepared and stored at room temperature for 12 h. The samples were treated and analyzed as described in the section on pretreatment of hair samples. Six samples were tested in parallel for three days continuously, and intraday accuracy and interday accuracy were calculated (Table II).
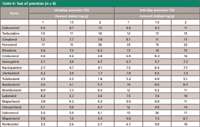
Table II: Test of precision (n = 6)
Recovery
A 500-mg blank hair sample was weighed following the assay method evaluation and results set out in the European Community guidelines. Hair samples at three standard concentrations (1.0, 1.5, and 2.0 ng/g) of clenbuterol-like β-agonist were prepared and stored at room temperature for 12 h. The samples were treated and analyzed as described in the section on pretreatment of hair samples. The content and recovery rates were calculated as recovery rate % = determined amount/added amount × 100% (Table III).
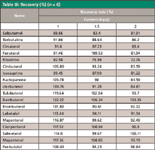
Table III: Recovery (%) (n = 6)
LOD and LOQ
The limits of detection (LODs), defined as the lowest value that differs from the blank with a 90% of confidence level, were all less than 0.5 ng/g for clenbuterol-like β-agonists. The limits of quantification (LOQs) were all less than 1 ng/g.
Application
All of the 20 samples of pig hair from the Shanghai hoggery and the 20 samples of human hair were collected randomly from the outskirts of Shanghai, treated as described above and analyzed by LC–MS-MS. The samples of pig hair were not found to contain any clen-buterol-like β-agonists. In the samples of human hair, two kinds of β-agonists were detected: clenbuterol and salbutamol. Six samples contained clenbuterol, with concentrations of 2.87, 1.65, 1.04, 1.30, 14.9, and 4.28 ng/g, respectively; and three samples contained salbutamol, with concentrations of 1.18, 1.69, and 1.55 ng/g, respectively.
Discussion
The Choice of Hydrolysis Method
Temperature of Hydrolysis
A 1-ng/mL standard solution of clen-buterol-like β-agonist was incubated in 0 °C, 40 °C, 50 °C, 60 °C, and 80 °C water baths overnight (18 h), respectively, and allowed to stand to room temperature, then analyzed by LC–MS-MS.The results showed that the content of some the β-agonists (such as salbutamol, fenoterol, isoxsuprine, ractopamine, bambuterol, brombuterol, clenpenterol, salmeterol, and penbutolol) decreased at temperatures greater than 50 °C. Therefore, 40 °C was used to hydrolyze the β-agonists present in hair.
Acid Hydrolysis or Alkaline Hydrolysis
The effects of acid and alkaline hydrolysis on 18 kinds of β-agonists were initially tested. Briefly, 1 ng/mL of β-agonist hydrochloric acid solution (0.1 mol/L) and 1 ng/mL β-agonist sodium hydroxide solution (1 mol/L) were prepared and each placed in a 40 °C water bath overnight for 18 h, removed, cooled to room temperature, and subjected to LC–MS-MS analysis. The results showed that concentrations of salbutamol, terbutaline, cimaterol, fenoterol, cimbuterol, ractopamine, isoxsuprine, and bambuterol decreased in alkaline solutions. Because the majority of β-agonists were in the form of hydrochloric salts and sulfate salts, acid conditions at 40 °C overnight had no effect. Because of the instability of some β-agonists under the alkaline conditions, acid hydrolysis was chosen for this study.
The Choice of Extraction Method
Extraction Number
Standard solution was added to five 500-mg portions of blank hair to prepare spiked hair samples of 1 ng/g. Then, 10 mL of 0.1 mol/L hydrochloric acid was added. Each solution was incubated at 40 °C overnight for 18 h, cooled to room temperature, shaken, centrifuged, and the hydrochloric acid hydrolysis solution removed. Five successive extractions using 5 mL of ammonium acetate solution (0.2 mol/L, pH 5.0) were performed on the samples. Each of the five extraction solutions was assayed by LC–MS-MS separately, and the chromatographic results are shown in Figure 2. The results showed that the third extraction solution was almost devoid of β-agonists. Thus, after hydrochloric acid hydrolysis of the hair, two extractions with ammonium acetate solution were chosen for this study.

Figure 2: TICs of five successive extractions.
Extraction Method
Standard solution was added to four 500-mg blank hair samples to prepare spiked hair samples of 1 ng/g. Then, 10 mL of 0.1 mol/L hydrochloric acid was added. The solution was incubated at 40 °C overnight for 18 h. The samples were cooled to room temperature, shaken, and centrifuged, and the hydrochloric acid extraction solution removed. To the sample, 5 mL of ammonium acetate solution (0.2 M, pH 5.0) was added, and two samples were shaken for 10 min, with another two samples being subjected to ultrasound for 10 min. LC–MS-MS was then carried out separately. The chromatographic results show that the extraction efficiency of shaking was slightly better than that of ultrasound; therefore, shaking was used in the study.
The Choice of Purification
The Choice of SPE
SPE is an effective method to purify various β-agonists. It is best applied on aniline and phenol and has various applications to β-agonist residue analysis. In this study, a mixed cation-exchange column was chosen, with double mechanisms of ion exchange and reversed-phase retention. The sample was put into the activated SPE column, and the β-agonist drugs were ionized at pH 5.0 and exchanged with SPE cation chromatography. After impurities were washed out, the β-agonist drugs became deionized and were washed out by ethyl acetate in saturated ammonia water. SPE columns (PCX, Agela, 30 mg, 3 mL) were chosed to purify the 18 kinds of β-agonist in the hair extraction solution for this study.
Eluent Choice
This study evaluated ethyl acetate and ether as eluents. A 1-ng/mL standard solution was added to the activated SPE column. Then ethyl acetate or ether, both in saturated ammonia water, was used to elute the β-agonist drugs retained by the column. The eluate was dried by nitrogen gas, dissolved again in ethyl acetate or ether in saturated ammonia water, and subjected to LC–MS-MS. Ethyl acetate offered better results than ether; thus, ethyl acetate was chosen for this study.
Verification
During sample analysis, retention time and the relative abundance ratio were important basis for qualitative analysis. In this study, two β-agonists, clenbuterol and salbutamol, were found in human hair. Their retention times and relative ion abundances were all within the appropriate range. Therefore, the analytical results were accurate and reliable.
Conclusion
In this study, a method for detecting 18 β-agonists in hair was established. To date, there has been no similar report in China and only one report internationally (5). This study simultaneously tested 22 β-agonists in hair using a strong alkaline solution to hydrolyze the hair. After examining the experimental results, it was found that a strong alkaline solution destroys β-agonists (such as salbutamol and others) in the hair, causing the concentrations of many kinds of β-agonists in the hair to decrease, and thus decreasing detection sensitivity. The concentration check of the method is 5 ng/g. To optimize the experimental conditions in this study, a mild acid solution was chosen to hydrolyze hair, and referring to the EU regulations, the lower concentration (1.0, 1.5, and 2.0 ng/g) hair samples were examined by the methodology. The detection limit was lower than 0.5 ng/g, and the established method had high sensitivity, strong specificity, rapid response, high accuracy, and was suitable for testing various kinds of β-agonists in hair.
References
(1) N.A. Botsoglou and D.J. Fletouris, Drug Residues in Foods: Pharmacology, Food Safety, and Analysis, (CRC Press, 2000).
(2) J-S. Lee, Y-M. Chiu, and T. Wang, Analysis of the Residues of the Animal Drug [M], (Shanghai Science & Technology Press, Shanghai, China, 2002), pp. 641–682.
(3) 9-EEC Directory 96/23/CE, Off. J. European Community, I.125 (1996) 10.
(4) EC Commission Decision 2002/657/EC, Off. J. European Communities L221/8 14 August, (2002).
(5) M.W.F. Nielen et al., Anal. Bioanal. Chem. 391, 199–210 (2008).
Wan-hua Yang is with the department of Pharmacy, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China. Wen-ting Ling, Qin Feng, and Gui-liang Chen are with the Shanghai Institute for Food and Drug Control, Shanghai, China.

Extracting Estrogenic Hormones Using Rotating Disk and Modified Clays
April 14th 2025University of Caldas and University of Chile researchers extracted estrogenic hormones from wastewater samples using rotating disk sorption extraction. After extraction, the concentrated analytes were measured using liquid chromatography coupled with photodiode array detection (HPLC-PDA).
Polysorbate Quantification and Degradation Analysis via LC and Charged Aerosol Detection
April 9th 2025Scientists from ThermoFisher Scientific published a review article in the Journal of Chromatography A that provided an overview of HPLC analysis using charged aerosol detection can help with polysorbate quantification.
Removing Double-Stranded RNA Impurities Using Chromatography
April 8th 2025Researchers from Agency for Science, Technology and Research in Singapore recently published a review article exploring how chromatography can be used to remove double-stranded RNA impurities during mRNA therapeutics production.
















