Using the Develosil UHPLC C18 and C30 for Oligonucleotide Analysis
Here we applied our newly developed UHPLC C18 and C30 columns to oligonucleotide analysis.
Reversed-phase HPLC is common for analysis of small to midsize molecules, but careful experimental design and selection of columns are both required for successful separation.
Here we applied our newly developed UHPLC C18 and C30 columns to oligonucleotide analysis. This new type of column is designed and optimized for the rapid separation of small to midsize molecules, utilizing 1.6-µm diameter particles with 11-nm diameter pores to achieve higher resolution. Theoretically, this pore size should allow separation of molecules of up to 25,000 kDa with this column.
Experimental Condition
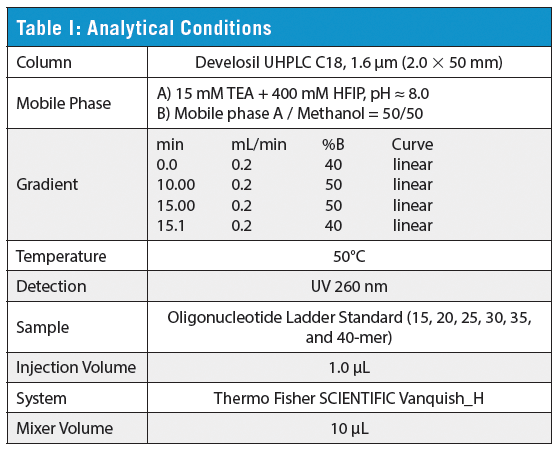
Table I shows the analytical conditions. For Develosil 1.6 µm UHPLC columns, C18 has a higher density of alkyl chains on the silica gel surface than C30. This results in the C18 columns having higher retention of hydrophobic compounds. The lower density of alkyl chains in the C30 column allows the mobile phase more access to the endcapped silica, which can influence the separation of hydrophilic compounds. Because of this, modulation of the pH and organic solvent content of the mobile phase can result in larger changes in retention of hydrophilic species such as oligonucleotides on C30 when compared to C18.
Results and Discussion
Figure 1 shows an oligodeoxythymidine ladder standard mixture (DNA ladder standard, oligos at 15, 20, 25, 30, 35, and 40 mer) separated using Develosil UHPLC C18 1.6 µm. The sample has higher concentrations at each 5-mer increment from 15-mer and up. This chromatogram shows each additional individual nucleotide length is a clearly separated peak.
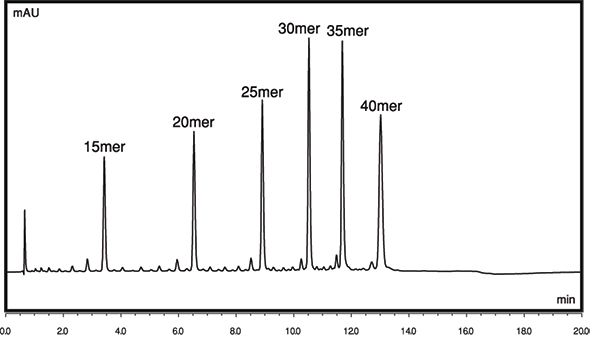
Figure 1: UHPLC C18 column separation of oligodeoxythymidine mixture.
We then applied Develosil UHPLC C30 1.6 µm to this analysis (Figure 2). Compared to C18, the C30 column retains nucleotides more strongly, leading to longer retention times under the same conditions. Increasing the final concentration of mobile phase B by 0.5% results in similar retention times to that of the C18 column. During the process of optimizing the mobile phase, we also found that small differences in mobile phase composition will affect retention time on C30 much more so than on C18.
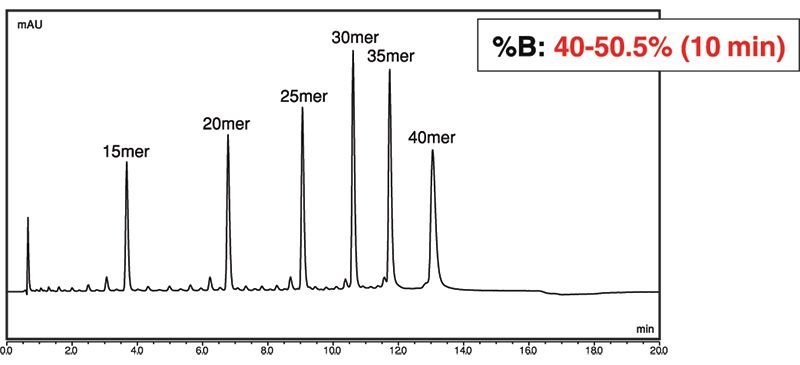
Figure 2: UHPLC C30 column separation of oligodeoxythymidine mixture
Conclusion
Using the 1.6 µm particle of Develosil UHPLC C18 and C30 showed clear and sharp peaks of oligonucleotides up to 40 mer. The resolution was higher on C30. This indicates that choosing different column lengths or mobile phase conditions can enable analysis of oligonucleotides 40 nucleotides or even longer.

Develosil USA
10060 Carroll Canyon Rd., Ste 100, San Diego, CA 92131
tel. (858) 800-2433
Website: www.develosil.us

Separating Impurities from Oligonucleotides Using Supercritical Fluid Chromatography
February 21st 2025Supercritical fluid chromatography (SFC) has been optimized for the analysis of 5-, 10-, 15-, and 18-mer oligonucleotides (ONs) and evaluated for its effectiveness in separating impurities from ONs.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)















