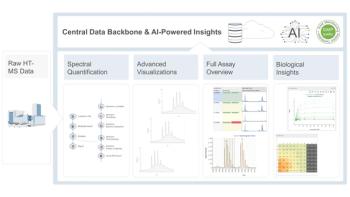
- The Application Notebook-02-01-2019
Simultaneous Analysis of Ten Water-Soluble Vitamins With a Polymer-Based Reversed-Phase Column– Shodex™ RSpak DE-413L
In this application, a simple method to simultaneously analyze various water-soluble vitamins was developed.
Vitamins are micronutrients essential for the metabolism of living organisms. Since humans cannot produce vitamins, the intake of vitamins must be part of their intake. There are many commercial foods and drinks supplemented with vitamins for nutrient enhancement purposes, including most processed foods.
Methods using microbiological assays, absorption spectrophotometry, and HPLC have been used to analyze vitamins, creating a long process. A typical HPLC method to separate and quantify vitamins can use an ODS column with an addition of ion-pair reagent. However, the ion-pair reagent tends to remain on the column and the flow-lines, resulting in increased background level and lowers the sensitivity.
Therefore, in this application, a simple method to simultaneously analyze various water-soluble vitamins was developed. Shodex DE-413L, a polymer-based reversed phase column, without the use of an ion-pair reagent. We further applied the developed method to quantify vitamins in a commercial multi-vitamin supplement.
Experimental
Ten vitamins (thiamin HCl, pyridoxine HCl, nicotinamide, ascorbic acid, nicotinic acid, calcium pantothenate, cyanocobalamin, folic acid, riboflavin, and biotin) were used as standards. A 4-mM standard solution was used for biotin and 2-mM standard solutions were used for other vitamins. More standars were dissolved in 250-mM phosphoric acid. Five levels of multi-vitamin calibration standards were prepared using standard solutions and 250-mM phosphoric acid. We used 250-mM of phosphoric acid to prevent the oxidation of ascorbic acid.
A Shodex RSpak DE-413L column (4.6 mm I.D. × 250 mm, 4 µm) was used with a PDA detector (190–400 nm). The eluent conditions were as follows: (A) 10 mM H3PO4 aq. / (B) CH3CN, linear gradient (high pressure); (B%) 0% (0 min) (→) 30% (5-10 min) (→) 0% (10.1–20 min). The column was kept at 50 °C and the flow rate was 1.0 mL/min.
Results and Discussion
Figure 1 shows the UV chromatograms of the standards. Peaks of the ten vitamins were fully resolved using the developed method. The UV absorbance was measured at 254 nm. However, since the absorbance of pantothenic acid and biotin at 254 nm were low, 210 nm was used for the measurement.
Figure 1: Sample UV chromatograms showing a simultaneous analysis of ten water-soluble vitamins.
This simple method using phosphoric acid and acetonitrile as the eluents demonstrated a successful simulated analysis of ten water-soluble vitamins in 20 min, including the column equilibration time.
We analyzed the extract of a commercial multi-vitamin supplement. We used a guard column (Shodex RSpak DE-G 4A) during the sample analysis (Figure 2).
Figure 2: Two UV chromatograms of the extract of a commercial multi-vitamin supplement
A method for simultaneous analysis of ten water-soluble vitamins was developed using the Shodex RSpak DE-413L column. The eluents used consisted of a mixture of an acid and acetonitrile. One sample measurement completes in 20 min.
The Shodex RSpak DE series provide a stable analysis even under highly aqueous eluent conditions, without the concern of column deterioration due to the polymer-based packing materials compared to using silica-based material.
References
(1) Xue Song Lianget al., Research on stability of synthetic folic acid, Advanced Materials Research Vol. 781-784 (2013) 1215-1218
Shodex™/Showa Denko America, Inc.
420 Lexington Avenue Suite 2335A, New York, NY 10170
tel. (212) 370-0033, X109
Website:
Articles in this issue
almost 7 years ago
Restek LC–MS/MS Analysis of Mycotoxins in Peanut Powder in 5.5 Minalmost 7 years ago
An Analysis of Amino Acids in Oxidized and Unoxidized Feed Samplesalmost 7 years ago
Add More Confidence to Your UHPLC–MS Analysesalmost 7 years ago
An Absolute Molar Mass Analysis of ChitosansNewsletter
Join the global community of analytical scientists who trust LCGC for insights on the latest techniques, trends, and expert solutions in chromatography.