Characterization of TSKgel FcR-IIIA-NPR HPLC Column by Top Down Mass Spectrometry
Monoclonal antibodies (mAbs) comprise the largest class of glycosylated protein therapeutics currently on the market, and glycosylation is known to be a major source of mAb heterogeneity. N-glycosylation of IgG-Fc of mAbs is known to impact drug therapeutic mechanism of action (MOA), thus monitoring glycan critical quality attributes (CQAs) is an essential part of biopharmaceutical development. Glycosylation is a critical factor in drug product solubility, kinetics, stability, efficacy, and immunogenicity. Analytical methods utilize a suite of chromatographic modes using high performance liquid chromatography (HPLC) to analyze glycosylation of both intact and digested protein molecules.
The TSKgel FcR-IIIA-NPR column is a high performance affinity chromatography column for the analysis of IgG glycoforms. The stationary phase utilizes a recombinant FcR-IIIA protein bound to a nonporous polymethacrylate polymer. The retention mechanism is based on the interaction between the FcR ligand and the sugar moieties attached to the ASN amino acid in the conserved region of the monoclonal antibody. The resulting elution profile of the glycoprotein mimics ADCC activity, which is correlated to the composition of the N-glycans.
The purpose of this study is to demonstrate the use of mass spectrometry to characterize the elution profile of a typical IgG1 molecule separation on a TSKgel FcR-IIIA-NPR column, and verify the observations that certain glycan structures impart higher activity to the monoclonal antibody, especially as it relates to the presence of terminal galactose sugars.
Experimental HPLC Conditions
Results and Discussion
Figure 1 demonstrates the separation of NIST mAb on the TSKgel FcR-IIIA-NPR column. IgG1 molecules yield this typical type of elution profile based on glycoform composition that is consistent with ADCC activity. This offers a fast orthogonal chromatographic method for determination of antibody activity and comparisons of antibody heterogeneity.
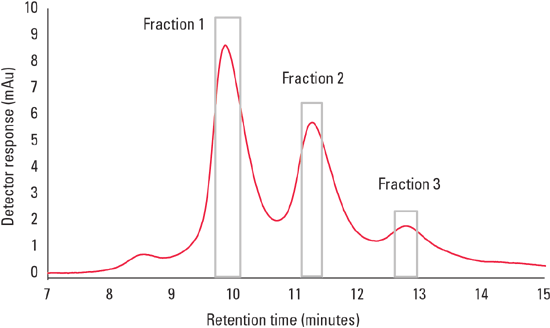
Figure 1: Zoomed view of the elution profile of NIST mAb onTSKgel FcR-IIIA-NPR. The boxes highlighting each peak represent fractions that were collected.
The three largest eluting peaks were collected and analyzed by offline mass spectrometry. Peak fractions were pooled from successive 25 µg on column injections, concentrated, and buffer exchanged to LC/MS grade water.
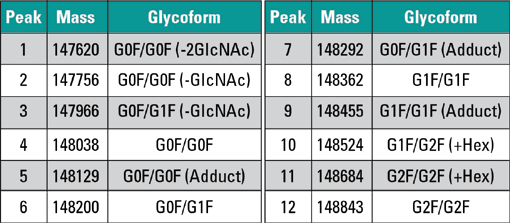
Figures 2 and 3 illustrate analysis of the NIST mAb standard compared against the collected peak fractions. It is observed that each peak has a unique composition of intact mAb glycoforms and that the selectivity of the stationary phase is based on the amount of terminal galactose units on the glycan moiety. This conclusion agrees with studies that show anitbodies with higher amounts of G1- and G2-containing sugars show greater ADCC activity. Because of some peak overlap in the initial separation, there is some overlap of different galactose-containing species in the MS profile, though the general trend between galactose and activity has been confirmed.
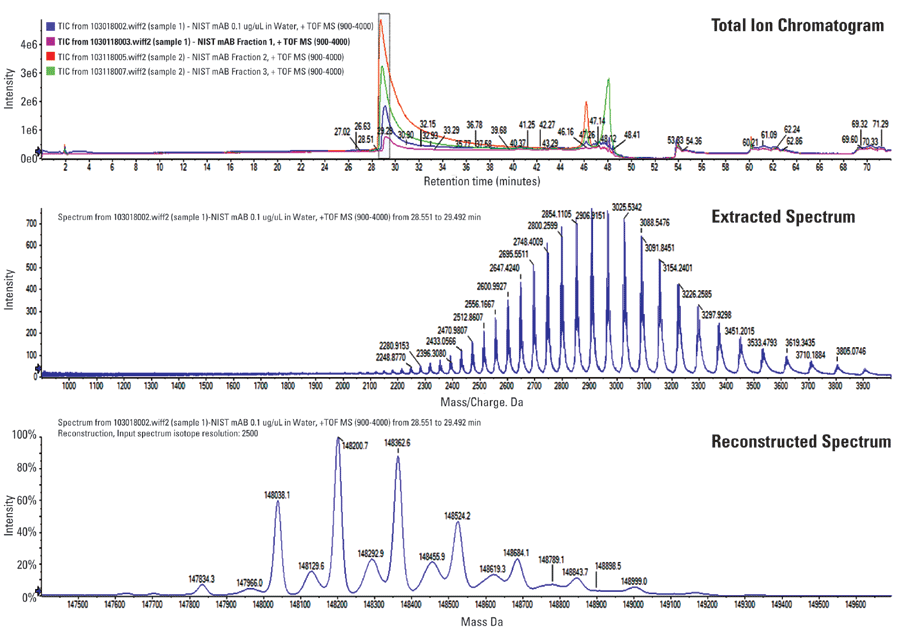
Figure 2: The TIC, extracted spectrum, and reconstructed spectrum for a NIST mAb control sample. The glycoforms observed for this sample are in agreement with accepted literature on characterization of this molecule.
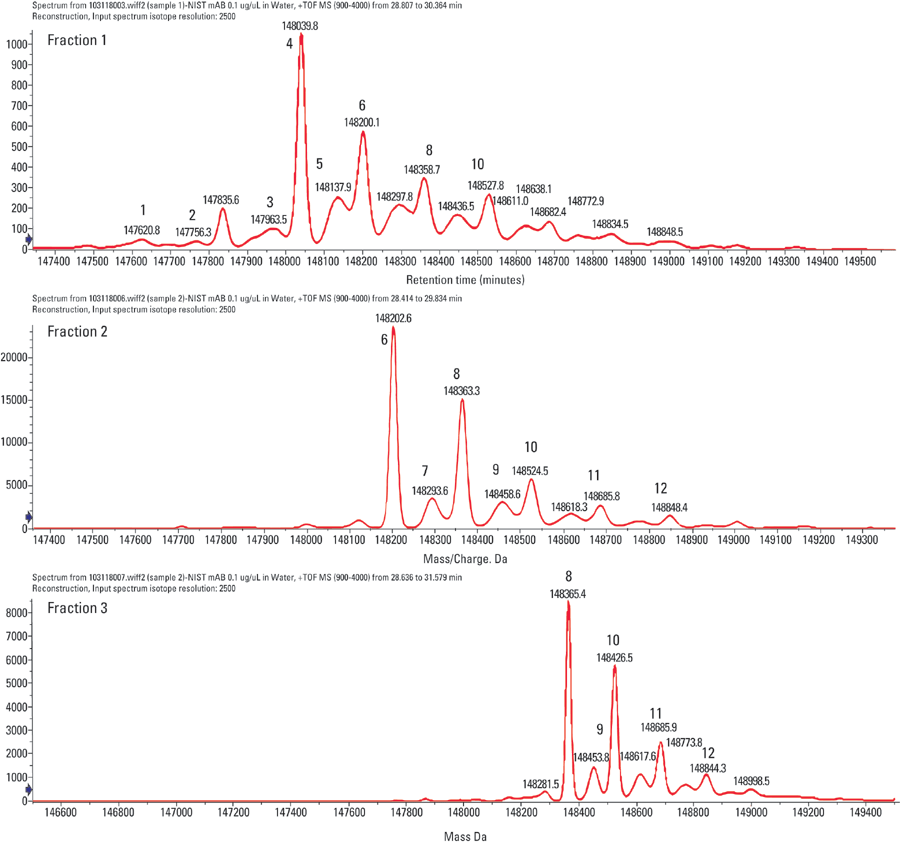
Figure 3: Reconstructed spectra for each of the isolated peak fractions, indicating that later eluting fractions have a greater proportion of termical galactose glycan sugars, consistent with observations of antibody activity and percentage of galactose.
Conclusions
The separation of an IgG1 molecule was demonstrated using the TSKgel FcR-lIIA-NPR column and peaks from that separation were characterized by high resolution mass spectrometry. The results support that the stationary phase selectivity is based on the same Fc-glycan/Fc receptor interaction as ADCC activity. The glycoform composition of each peak is consistent with previous published observations on the activity of N-glycan sugars with higher amounts of terminal galactose.
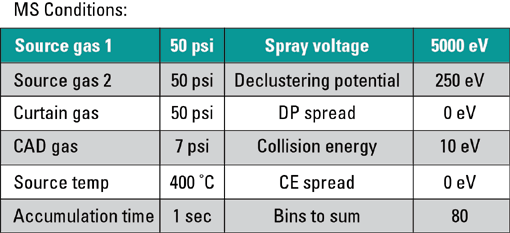
MS Conditions
This application demonstrates the efficacy of this approach and characterization data that demonstrate the proof of concept of this chromatographic technique for a fast orthogonal analysis to evaluate mAb ADCC activity, potentially for early cell line development, bioreactor modeling and lot-to-lot comparability of therapeutic antibodies.
Tosoh Bioscience and TSKgel are registered trademarks of Tosoh Corporation.
Nexera is a registered trademark of Shimadzu Corporation.

Tosoh Bioscience LLC
3604 Horizon Drive, Suite 100, King of Prussia, PA 19406
tel. (484) 805-1219, fax (610) 272-3028
Website: www.tosohbioscience.com


.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)











