The Use of Extraction Technologies in Food Safety Studies
LCGC North America
Traditional extraction methods for food samples, such as liquid-liquid extraction and Soxhlet extraction, are often time-consuming and require large amounts of organic solvents. Therefore, one of the objectives of analytical food safety studies currently has been the development of new extraction techniques that can improve the accuracy and precision of analytical results and simplify the analytical procedure.
Traditional extraction methods for food samples, such as liquid–liquid extraction and Soxhlet extraction, are often time consuming and require large amounts of organic solvents. Therefore, one of the objectives of analytical food safety studies currently has been the development of new extraction techniques that can improve the accuracy and precision of analytical results and simplify the analytical procedure.
Food safety has become a top concern in our society. In general the public is increasingly concerned about the safety of the food products they consume every day as more and more food contamination incidents and widespread recalls arise. Such incidents have alerted the authorities and the public that more efforts and deeper investigations are needed. As a result, reliable and efficient methods for food safety analyses are required. Even with modern detection techniques, because of the low concentrations of contaminants and complicated food matrices, efficient sample preparation is necessary. Traditional extraction methods for food samples, such as liquid–liquid extraction (LLE) and Soxhlet extraction, are often time-consuming and require large amounts of organic solvents. Therefore, one of the objectives of analytical food safety studies currently has been the development of new extraction techniques that can improve the accuracy and precision of analytical results and simplify analytical procedures.
Because of increased concerns for food safety, attention is given to developing methods for determination of contaminants and other harmful substances from food samples. The analysis of food samples is usually a complicated procedure involving many steps. It requires extensive sample extraction before further analysis. Sample extraction is a crucial step in food sample analysis because it can affect the concentration of the analyte and the cleanliness of the sample. Traditional sample extraction techniques used in food safety studies are based on the suitable choice of solvents and the use of heat and agitation to improve the solubility of the desired compounds and the mass transfer (1), like in Soxhlet, liquid–liquid, and shake-flask extraction. Some of the traditional extraction techniques can require a great deal of time-Pedersen and Olsson (2) performed Soxhlet extraction of acrylamide from potato chips, and it took seven days to get a complete extraction. Frenich and coworkers (3) reported a method for the determination of residues of organochlorine and organophosphorus pesticides using Soxhlet extraction. This extraction method involved laborious steps with the use of large amounts of solvent. Analysis of polychlorinated biphenyls (PCBs), polychlorinated dibenzo-p-dioxins (PCDDs), and polychlorinated dibenzofurans (PCDFs) in butter based on three different liquid–liquid extraction methods was studied by Ramos and coworkers (4). The reported methods also involved time-consuming and large solvent consumption steps. These traditional extraction techniques are quite laborious, time-consuming, and involve large quantities of organic solvents, which are flammable, expensive, and generate hazardous waste.
In recent years, several new extraction techniques emerged as alternatives to the conventional sample preparation methods, including microwave-assisted extraction (MAE), ultrasound-assisted extraction (UAE), accelerated solvent extraction (ASE) (also known as pressurized solvent extraction [PSE]), supercritical fluid extraction (SFE), and solid-phase microextraction (SPME). These new extraction techniques have numerous advantages over traditional extraction methods, including shortened extraction times, reduced solvent consumption, reduced cost, and automation. In this column, we will provide an overview of these newer extraction techniques that have been applied to food safety studies.
Liquid–Liquid Extraction
Liquid–liquid extraction is the most widely used method for the extraction of analytes from aqueous food samples. In LLE, the sample is distributed or partitioned between two immiscible solvents in which the analyte has different solubilities. The solution containing the analyte must be immiscible with the solvent used to extract the analyte. The main advantages of this method are the wide availability of solvents and the use of low-cost apparatus. However, low recoveries, limited selectivity, and time-consuming procedures limit LLE. A variety of microscale variants of LLE have been reported and food applications of these have been recently reviewed (5).
Solid-Phase Extraction
In solid-phase extraction (SPE), the sample passes over the stationary phase (solid phase). The analytes separate according to the degree to which each component is partitioned or adsorbed by the stationary phase. The analytes may favorably adsorb to the solid phase or they may remain in the liquid phase. If the analytes are adsorbed, a stronger eluting solvent selectively desorbs the analytes. If the analytes remains in the liquid phase, they can be collected and prepared properly for further analysis, provided the interferents are bound to the solid-phase sorbent.
Effective separation by SPE can be achieved by choosing suitably selective solid-phase sorbent and eluting solvents. With proper selection of the sorbent and solvents, SPE is capable of being used for gases, solids, and liquids. However, the primary area of application of SPE is in the selective extraction and enrichment of liquids samples. SPE is used widely in the environmental, pharmaceutical, biological, clinical, forensic science, and food and beverage areas.
SPE is also used to concentrate and clean a sample before using a chromatographic or other analytical method. SPE has very extensive applications in food safety studies because of its low cost, good selectivity, small solvent consumption, and high recovery. However, long sample preparation times and multistep procedures are among its disadvantages.
Solid-Phase Microextraction
SPME, developed by Pawliszyn and coworkers (6) in 1990, involves the use of a fiber coated with suitable stationary phase extracting material for the removal of analytes of interest from the sample. The sample molecules adsorb onto the fiber and subsequently desorb into the gas chromatograph’s injection port for analysis. It is a simple, fast, inexpensive, and efficient extraction method that has been applied to both headspace and aqueous sample analysis with great sensitivity and selectivity (7).
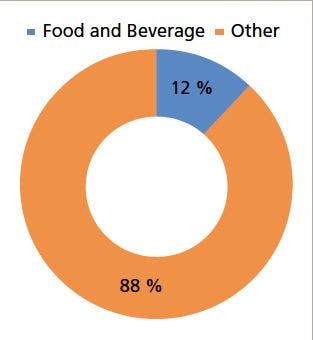
SPME is most effective when coupled to gas chromatography (GC). It has also been used with high performance liquid chromatography (HPLC) separations (8). Figure 1 shows the SPME device (9). It consists of a fiber bonded to a stainless steel plunger and installed in a holder. The fiber, which is coated with a suitable stationary phase, is immersed in the sample or exposed to the headspace above the sample. Analytes in aqueous samples are extracted by direct immersion, where analytes partition between the aqueous sample and the fiber coating. When equilibrium is reached, the fiber is removed and exposed to the injection port of a gas chromatograph for analysis. Headspace analysis can be used for the extraction of volatile or semivolatile analytes from solid, liquid, or gaseous samples. In the headspace extraction mode, the analytes first partition between the sample and the headspace, then the analytes are adsorbed by the fiber, which is then inserted directly into the injection port of a GC system (10).
Figure 1: Schematics of: (a) the fiber SPME extraction procedure, (b) thermal desorption in a GC injection port and (c) solvent desorption using an SPME interface.
Since SPME is an equilibrium extraction technique, several factors influence the extraction efficiency, such as fiber-coating thickness and characteristics, sample size, vial size, adsorption and desorption conditions (temperature and time) (10). To achieve successful quantitative analysis, it is vital that each of these variables is constant between analyses.
SPME has become increasingly popular in the analysis of volatile and semivolatile compounds because of its advantages over conventional extraction methods. It is a simple, effective, and low-cost technique. The extraction combines sampling, isolation, and concentration in one step. SPME is also considered environmentally friendly because of the elimination of organic solvents. The technique has been widely applied to food samples (7,11–13). One of these recent reviews (11) provided a summary of fiber coatings, while another (13) discussed techniques that are considered variants of SPME.
Shake-Flask Extraction
The most common approach for extraction from solids is conventional liquid-solid extraction, in the form of shake-flask extraction. Shake-flask extractions can be easily performed by putting a sample into a flask, adding solvent, and agitating. After extraction, the extract is separated from the solid residue by filtration. Shake-flask extraction requires minimal glassware, small amounts of organic solvent, and is comparatively fast (10–50 min). It is one of the oldest and most widely used extraction methods. However, because of its poor recovery and low efficiency, the application is limited.
Soxhlet Extraction
Soxhlet extraction is a traditional extraction technique for many food samples. It was originally designed for the extraction of a lipid from a solid material by Franz von Soxhlet in 1879 (14). The technique uses a specialized piece of glass apparatus where the solid sample is placed and is semicontinuously extracted with a sub-boiling solvent. Though Soxhlet extraction is simple, standard, and robust, there are disadvantages (15). Soxhlet extraction usually requires long extraction times (8–24 h) and large amounts of solvent. The operation lacks automation, but several samples can be extracted in parallel.
QuEChERS
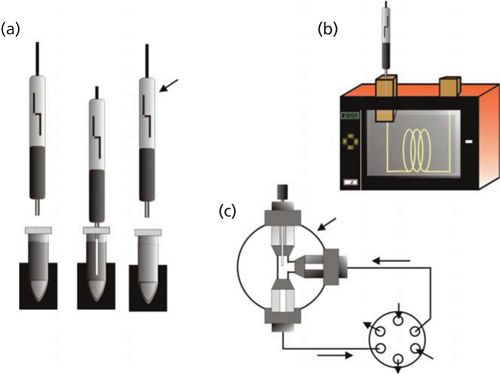
QuEChERS (quick, easy, cheap, effective, rugged, and safe) extraction has become a very attractive sample extraction method for various food samples. This method was developed by Lehotay and Anastassiades in 2003 for the analysis of pesticides in vegetables and fruits (16). Now, QuEChERS has been widely used in pharmaceutical, clinical, and environmental analysis including steroids, hormones, acetaminophen, acrylamide, perfluorinated compounds, polycyclic aromatic hydrocarbons, alkaloids, mycotoxins, and other applications. Overall, this procedure has two main steps: Extraction with a solvent and partitioning salts, and clean up with dispersive solid-phase extraction (dSPE). Figure 2 displays the sequence of events in QuEChERS in the original and the AOAC and European official methods (17). The QuEChERS method has many advantages over traditionally used techniques. QuEChERS provides accurate analytical results with high recoveries, saves time and labor, reduces hazardous solvent consumption and waste disposal, and uses less laboratory glassware with a minimal number of steps. Rajczak and Tuzimski (18) recently reviewed QuEChERS, including food applications.
Figure 2: Steps in the original and official versions of QuEChERS sample preparation for pesticide residues in food commodities.
Ultrasound-Assisted Extraction
Ultrasound-assisted extraction is also employed in food safety studies for the extraction of contaminants or bioactive components from food materials. The principle of UAE is based on the propagation of ultrasound pressure waves and resulting in a cavitation phenomena. Ultrasound waves are elastic waves that have a frequency above the threshold of human hearing, approximately 20 kHz. The extraction mechanism involves two steps, diffusion through the cell walls and releasing the cell content after the walls are disrupted (19). In one configuration, the sample is immersed in an ultrasonic bath with a solvent and subjected to ultrasonic radiation. Higher energy extractions use a horn or probe device. Ultrasound waves create bubbles in the solvent and produce high local negative pressure that can cause the collapse of cavitation bubbles. The collapse of cavitation bubbles near cell walls produces cell disruption, and as a result, solvent penetrates into the cells and causes the release of extractable compounds. The ultrasound waves can also facilitate the diffusion process and increase mass transfer.
UAE can reduce extraction time and solvent consumption, thus resulting in higher extraction rates and good extraction efficiency. Compared to other extraction techniques, UAE is simple, fast, productive, inexpensive, and capable of operating with many samples at one time. UAE usually provides good results for food samples (20–22). The benefits for using UAE for food samples include enhancement of extraction yield or rate and extraction of heat-sensitive bioactive and food components under lower processing temperatures. Food components such as antioxidants, phenols, aromas, carotenoids, anthocyanins, and oils can be isolated from fruits and vegetables, herbs and spices, and seeds using UAE (22).
Microwave-Assisted Extraction
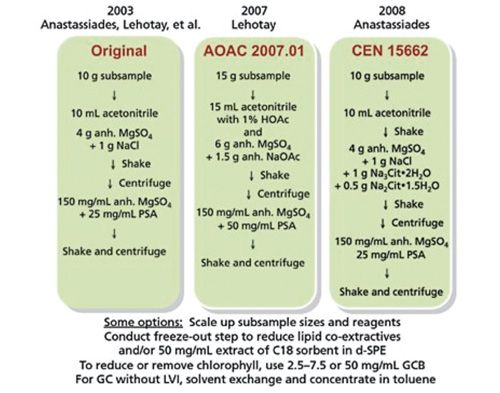
Microwave-assisted extraction is an extraction technique that combines microwave and traditional solvent extraction. Its use in food safety analysis has become one of the more common and low-cost extraction methods today. Typically, a microwave system includes a microwave power generator, a waveguide for transmission, a resonant cavity, and a power supply (23). The microwave power generator is a magnetron. At the common microwave frequency of 2.45 GHz, electromagnetic energy is conducted from the magnetron to the cavity using a waveguide. The sample and solvent placed inside the resonant cavity is subjected to microwave energy. This arrangement is outlined in Figure 3 (24). After typically 5–30 min, the extraction is complete, and the extract can be filtered and prepared for analysis.
Figure 3: Schematic diagram of instrumentation for microwave-assisted extraction (24).
Compared to other extraction techniques, an important advantage of MAE is the extraction rate acceleration resulting from the high temperatures employed. Therefore, short extraction times are obtained. Other advantages include reduced solvent consumption and improved extraction yield and product quality. On the other hand, disadvantages include an additional filtration step needed to remove the solid residue after the extraction, the poor efficiency of microwaves when the solvents are nonpolar and volatile, and the use of high temperatures that might degrade heat-sensitive compounds. The stated requirement that analytes or samples must be polar to absorb microwave energy is usually ignored with food analysis because of the typically high water content.
MAE has been applied to a diverse range of sample types (soils, sediments, sewage sludge, plants, food). MAE is employed extensively in the extraction of pesticides, pigments, bioactive compounds from vegetables, plants, and natural products as an alternative to traditional techniques of extraction (25,26).
Supercritical Fluid Extraction
Supercritical fluid extraction uses a fluid phase having unique properties between a gas and a liquid to effect the solubilization of solutes. Compared to traditional liquid solvents, supercritical fluids have lower viscosities and high diffusivities, thus allowing more-efficient mass transfer of solutes from sample matrices. SFE can be operated in two modes, off-line and on-line (27). In the on-line mode, the SFE instrument is coupled directly to the analytical instrument, such as with SFE–GC. Off-line SFE focuses on the sample preparation only, for analytical purposes or on a larger scale to either remove unwanted components from a product or collect desired components (28).
An SFE system contains reservoirs for the supercritical fluid and cosolvent, a thermostated extraction cell, a restrictor to maintain flow and pressure, and a collection vial. Typically, the supercritical fluid is pumped to a heating zone, where it is heated to supercritical conditions. It then passes into the extraction cell, where it rapidly diffuses into the sample and dissolves the components to be extracted. The dissolved components flow from the extraction cell into a collection vial. The supercritical fluid can then be condensed and recycled, or discharged to atmosphere.
The most commonly used supercritical fluid is carbon dioxide, which has a critical point of 31.3 °C and 72.8 bar. This low critical temperature and pressure allows extraction to occur near room temperature and at a mild pressure. Carbon dioxide is inexpensive, nontoxic, nonflammable, inert, and a good solvent for nonpolar molecules. In general, supercritical carbon dioxide extraction has a very wide range of applications, such as in food, cosmetics, pharmaceutical, environmental, and other related industries. Pesticides, organic pollutants, fats and lipids, flavors, and natural bioactive components are all classes of compounds that can be separated and extracted from food sample (29). One attractive feature for the use of carbon dioxide in food analysis is that it is “generally regarded as safe” by the U.S. Food and Drug Administration.
Accelerated Solvent Extraction
Accelerated solvent extraction is a fast and automatic sample extraction technique that uses elevated temperatures and pressures with liquid solvents to obtain fast and efficient extractions. It allows a high extraction efficiency with a small volume of solvent (10–40 mL) and a short extraction time (5–20 min).
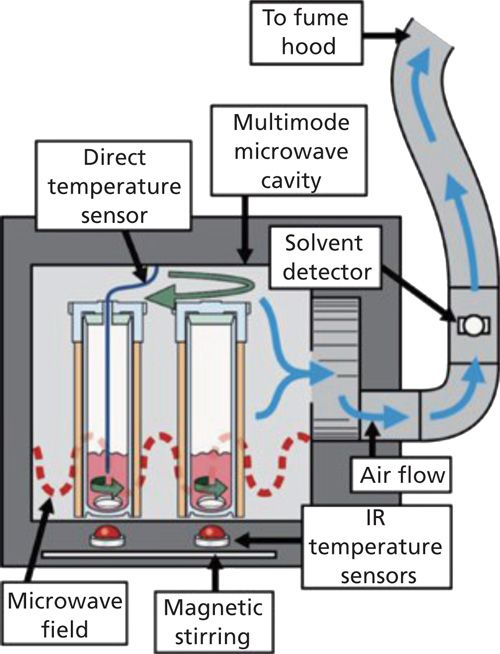
ASE is mostly applicable to solid or semisolid samples that can be held in the extraction cell during extraction. A schematic of the ASE apparatus is presented in Figure 4 (30). With ASE, a solvent or a mixture of solvents is pumped into an extraction cell containing the sample, which is then brought to elevated pressure and temperature for extraction. Following extraction, the sample is purged with compressed gas and prepared for analysis. Application of ASE in food safety studies has been reported for the extraction of various compounds and contaminants like residual pesticides, fats and lipids, food additives, and microbial contaminants in food samples (31).
Figure 4: Schematic diagram of accelerated solvent extraction instrumentation.
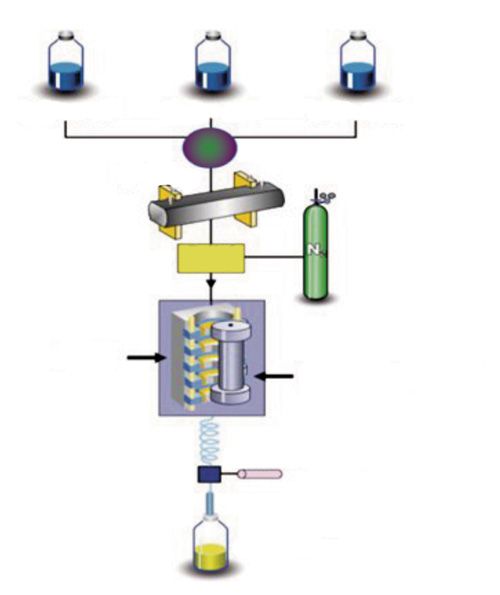
Optimization of various parameters in ASE, including solvent, temperature, pressure, extraction cycles, and time, is necessary to achieve good efficiency, quantification, and reproducibility. For an efficient extraction, the solvent must be able to solubilize the desired analyte while keeping the sample matrix intact. Most organic solvents and buffered aqueous solutions can be used in ASE, so the need for extraction and the cost of the solvent should be considered when developing a method. ASE uses high temperatures to accelerate the extraction processes. As the temperature is increased, solvent diffusivity increases and viscosity decreases, increasing the extraction rate. The temperatures used in most ASE applications are in the 50–150 °C range. Changing pressure has little impact on ASE extraction, as the main effect of pressure is to maintain the solvent in its liquid state. Most accelerated solvent extractions use 1500 psi as the standard operating pressure. Static extraction cycles are used to introduce fresh solvent during the extraction process, which maintains favorable extraction equilibrium. Time is also optimized to obtain a complete and efficient extraction.
Thermal Desorption
Thermal desorption is a well-known sample introduction technique for GC determination of volatile or semivolatile organic compounds. For gaseous samples, volatile organic compounds are collected onto a sorbent, then thermally desorbed for GC analysis, while volatile and semivolatile analytes in solid samples can be determined directly by thermal desorption.
Thermal desorption has numerous benefits for analysis of trace-level volatile and semivolatile organic compounds. Thermal desorption performs sample collection and concentration at the same time. The use of sorbents enables accurate and efficient analyses of volatile organic compounds in large sample volumes even at low concentration. Thermal desorption uses heat instead of solvent to desorb analytes from the sorbent and transfers the entire sample to a GC system for analysis. This technique enables a complete, fast and solvent-free desorption of the analytes. Thermal desorption is a flexible, efficient, and convenient sample introduction method. It has myriad applications, including fragrances and flavors.
Static Headspace Extraction
Headspace extraction is usually defined as a vapor-phase extraction, involving the partitioning of analytes between a nonvolatile liquid or solid phase and the vapor phase above the liquid or solid. In this process, the sample is placed in a sealed glass vial with a septum-lined cap. The vial is then heated to a specific temperature so that the volatile compounds diffuse into the headspace above the sample. After the equilibrium is reached, the analytes in the headspace are collected with a gas-tight syringe and injected into a GC system for analysis. The extraction of volatile and semivolatile organic compounds in solid, liquid, and gas samples can be achieved by headspace analysis. This extraction technique is simple and fast, and it can provide acceptable sensitivity. Common applications include analyses of flavor compounds in beverages and food products.
Purge-and-Trap Concentration
The purge-and-trap method is a dynamic headspace technique that involves the purging of inert gas through a liquid or solid sample, followed by trapping of the volatile analytes on a sorbent and desorption into a GC system for separation and identification. This method uses the inert gas to strip the volatile analytes from the sample matrix and concentrate them on a sorbent. Purge-and-trap concentration reduces matrix effects and increases sensitivity. This sampling method has been used extensively in food analysis (32,33).
Conclusions
The various extraction methods described here provide an overview of methods that are widely used in food safety analysis. Conventional methods such as Soxhlet extraction, liquid–liquid extraction, and shake-flask extraction are laborious, require the use of large amount of solvents and tedious extraction steps, and have limited applications. Modern extraction methods such as those presented here have numerous advantages when compared to the traditional methods, such as shortened extraction time, reduced solvent and energy consumption, and improved extraction efficiency. They are usually considered to be green techniques and have been used extensively for determination of various contaminants and harmful substances in food samples.
References
- L. Wang and C.L. Weller, Trend Food Sci. Technol.17,300–312 (2006).
- J.R. Pedersen and J.O. Olsson, Analyst 128, 332–334 (2003).
- A. Garrido Frenich, J.L. Martínez Vidal, A.D. Cruz Sicilia, M.J. González Rodríguez, and P. Plaza Bolaños, Anal. Chim. Acta 558, 42–52 (2006).
- L. Ramos, E. Eljarrat, L.M. Hernández, J. Rivera, and M.J. González, Chemosphere 38, 3141–3153 (1999).
- A. Lawal, G.H. Tan, and A.M. Ali, J. AOAC Intl.99, 1383–1394 (2016).
- C.L. Arthur and J. Pawliszyn, J. Anal. Chem.62, 2145–2148 (1990).
- H. Kataoka, H.L. Lord, and J. Pawliszyn, J. Chromatogr.A880, 35–62 (2000).
- J. Aulakh, A. Malik, V. Kaur, and P. Schmitt-Kopplin, Crit. Rev. Anal. Chem.35, 71–85 (2005).
- M.E.C. Queiroz and F.M. Lanças, LCGC Europe 18, 145–154 (2005).
- A.J. King, J.W. Readman, and J.L. Zhou, Environ. Geochem. Health25, 69–75 (2003).
- C.-H. Xu, G.-S. Chen, Z.-H. Xiong, Y.-X. Fan, X.-C. Wang, and Y. Liu, TrAC Trend. Anal. Chem. 80, 12–29 (2016).
- J. Li, Y.-B Wang, K.-Y. Li, Y.-Q. Cao, S. Wu, and L. Wu, TrAC Trend. Anal. Chem. 72, 141–152 (2015).
- T.A. Souza-Silva, E. Gionfriddo, and J. Pawliszyn, TrAC Trend. Anal. Chem.71, 236–248 (2015).
- F. Soxhlet, Polytechnisches J.232, 461–465 (1879).
- M.L. De Castro, and L. Garcıa-Ayuso, Anal. Chim. Acta369, 1–10 (1998).
- M. Anastassiades, S.J. Lehotay, D. Štajnbaher, and F.J. Schenck, J. AOAC Intl. 86, 412–431 (2003).
- S.J. Lehotay, R.E. Majors, and M. Anastassiades, LCGC North Am.28, 504–516 (2010).
- T. Rejczak and T. Tuzimski, Open Chem. 13, 980–1010 (2015).
- D. VeliÄkoviÄ, D. MilenoviÄ, M. RistiÄ, and V. VeljkoviÄ, Ultrasonics Sonochem. 13, 150–156 (2006).
- Z. J. Dolatowski, J. Stadnik, and D. Stasiak, Acta Sci. Pol., Technol. Aliment6, 89–99 (2004).
- K. Vilkhu, R. Mawson, L. Simons, and D. Bates, Innov. Food Sci. Emerg. Techn.9, 161–169 (2008).
- F. Chemat, N. Rombaut, A.-G. Sincaire, A. Meullemiestre, A.-S. Fabiano-Tixier, and M. Abert-Vian, Ultrasonics Sonochem.34, 540–560 (2017).
- E. Thostenson, and T.-W. Chou, Composites Part A: Appl. Sci. Manufac.30, 1055–1071 (1999).
- B. McManus, M. Horn, B. Lockerman, S. Smith, and G. LeBlanc, LCGC North Am.31(11), 22–27 (2013).
- H. Wang, J. Ding, and N. Ren, TrAC Trend. Anal. Chem. 75, 197–208 (2016).
- L. Angilillo, M.A. Del Nobile, and A. Conte, Curr. Opin. Food Sci. 5, 93–98 (2015).
- J. W. King, J. Chromatogr. Sci.27, 355–364 (1989).
- C. Turner, C.S. Eskilsson, and E. Björklund, J. Chromatogr. A947, 1–22 (2002).
- M. Herrero, J.A. Mendiola, A. Cifuentes, and E. Ibáñez, J. Chromatogr. A1217, 2495–2511 (2010).
- A. Kettle, LCGC North Am.31(11), 28–33 (2013).
- P. Vazquez-Craig and Y. Rico, TrAC Trend. Anal. Chem. 71, 55–64 (2015).
- J.G. Wilkes, E.D. Conte, Y. Kim, M. Holcomb, J.B. Sutherland, and D.W. Miller, J. Chromatogr. A880, 3–33 (2000).
- L. Zoccolillo, L. Amendola, C. Cafaro, and S. Insogna, J. Chromatogr. A1077, 181–187 (2005).

Changling Qiu is a post-doctoral associate in the Department of Chemistry and Biochemistry at the University of Texas at Arlington, where she is investigating vacuum-UV detection for capillary GC. She earned degrees in food engineering at universities in China and completed her PhD in chemistry at South Dakota State University, studying extraction development for food safety applications.
Douglas E. Raynie

“Sample Prep Perspectives” editor Douglas E. Raynie is Department Head and Associate Professor in the Department of Chemistry and Biochemistry at South Dakota State University. His research interests include green chemistry, alternative solvents, sample preparation, high resolution chromatography, and bioprocessing in supercritical fluids. He earned his PhD in 1990 at Brigham Young University under the direction of Milton L. Lee.

Analytical Challenges in Measuring Migration from Food Contact Materials
November 2nd 2015Food contact materials contain low molecular weight additives and processing aids which can migrate into foods leading to trace levels of contamination. Food safety is ensured through regulations, comprising compositional controls and migration limits, which present a significant analytical challenge to the food industry to ensure compliance and demonstrate due diligence. Of the various analytical approaches, LC-MS/MS has proved to be an essential tool in monitoring migration of target compounds into foods, and more sophisticated approaches such as LC-high resolution MS (Orbitrap) are being increasingly used for untargeted analysis to monitor non-intentionally added substances. This podcast will provide an overview to this area, illustrated with various applications showing current approaches being employed.
Thermodynamic Insights into Organic Solvent Extraction for Chemical Analysis of Medical Devices
April 16th 2025A new study, published by a researcher from Chemical Characterization Solutions in Minnesota, explored a new approach for sample preparation for the chemical characterization of medical devices.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)