Top 10 HPLC and UHPLC Column Myths
LCGC Asia Pacific
In any field, there are "misconceptions" or "myths" that arise and are perpetuated and passed on to the next generation. These myths are often driven by a lack of understanding of the real issues by practitioners. In the first of a two-part feature from Ron Majors, the top 10 high performance liquid chromatography (HPLC) column myths are presented and attempts are made to demystify them by offering some evidence that they are untrue. This part will feature myths 10 to six.
Webster's New Collegiate Dictionary defines a myth as "an ill-founded belief held uncritically, especially by an interested group." Could that group be misinformed chromatographers? In the first of a two-part feature from Ron Majors, the top 10 high performance liquid chromatography (HPLC) column myths are presented and attempts are made to demystify them by offering some evidence that they are untrue. This part will feature myths 10 to six. Since ultrahigh-pressure liquid chromatography (UHPLC) has come about, new myths are popping up and these shall also be dealt with here.
In any field there are often "misconceptions" or "myths" that are perpetuated and passed on to the next generation. These myths are often driven by a lack of understanding by practitioners of the real issues, and can change as time moves on. Originally, seven years ago, in a "Column Watch" instalment (1), the 10 most popular myths of the time around high performance liquid chromatography (HPLC) column technology were demystified by discussing the issues at hand. Because HPLC is approaching its 50th year, many column myths have already been passed down to two generations of liquid chromatographers. Recently, ultrahigh-pressure liquid chromatography (UHPLC) has come into its own and a new set of myths are arising. The purpose of this instalment of "Column Watch" is to revisit and update readers on the most popular column myths of today and try to dispel some of these myths before they get perpetuated. This column is an adaptation of an oral presentation at the HPLC2013 conference in Amsterdam, the Netherlands (2). In keeping with the "countdown theme," I will start with number 10 and work my way up to the top myth.
Myth 10: Air Will Kill an HPLC Column
False: HPLC and UHPLC columns are shipped with plugs of either stainless steel or polymeric construction installed at both end. Users are told that a column should always be capped tightly after the column is disconnected from the instrument. The thought is that large amounts of air can get inside the column, perhaps damaging the packing material, causing bubbles in the detector flow cell when installed into the HPLC system in the future, and maybe disrupting the packed-bed morphology. One should first realize that the tiny hole in the endfitting is less than 0.02 in. in diameter and therefore has an extremely small cross-sectional area. If left open, the small amount of air that diffuses into the column could hardly cause irreparable damage. Depending on the volatility of the solvent used to store the column, there could be some evaporation near the end of the column. But large quantities of air would have a hard time diffusing through the microparticles in the packed bed seeing that we need thousands of pounds per square inch of pressure to push liquid mobile phases through these micrometre-sized particles. The small amount of air that could conceivably enter into the ends of the column would be immediately dissolved once the system was pressurized or, at least be flushed out in the initial pressurization in a short time and should not cause any problems with the chromatography later on. However, if you feel more secure by capping the endfittings, by all means do so.
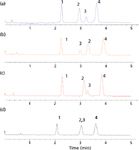
Figure 1: Chromatograms obtained using C18 bonded phases with the same base material but different chemistries: (a) Zorbax Eclipse Plus C18 (different surface treatment for same base silica, double endcapping, same bonding chemistry as Eclipse XDB-C18); (b) Zorbax StableBond SB-C18 (same base silica, sterically protected C18 phase, no endcapping); (c) Zorbax Eclipse XDB-C18 (same base silica, monomeric bonding chemistry, double endcapping); (d) Zorbax Extend-C18 (same base silica, bidentate bonding chemistry, double endcapping). Column dimensions: 50 mm à 4.6 mm, 1.8-µm dp; mobile phase: 69:31 acetonitrile-water; flow rate: 1.5 mL/min; temperature: 30 °C; detection: single-quadrupole electrospray ionization MS, positive mode scan. Peaks: 1 = anandamide, 2 = palmitoylethanolamide, 3 = 2-arachinoylglycerol, 4 = oleoylethanolamide.
Myth 9: All C18 (L1) Columns Are the Same
False: All of our HPLC column surveys have shown that C18 is, by far, the most popular bonded phase in existence (3). Because pharmaceutical manufacturers were the earliest adopters of HPLC, the United States Pharmacopeial Convention (USP), not wanting to favour any particular manufacturer of HPLC columns, developed a classification system that gave a generic description for each type of bonded phase column that was submitted under a new drug application. For HPLC columns, an "L" designation was given, and because C18 is used for a majority of submittals, its designation was "L1." As additional phases came along, they were given their own "L" number such as C8 (L7), CN (L10), phenyl (L11), and so on. The implication with this system was that each C18 column that was submitted also designated as L1, was the same as the last L1 column. Unfortunately, this system proved to be unreliable because columns from different manufacturers, produced from different base silicas and bonded with different silane reagents using different synthetic routes, were not chromatographically the same and one could therefore not be substituted for another. With more than 800 different L1 columns introduced into the marketplace, it has proven to be a confusing system. Several approaches, including the use of the hydrophobic subtraction model (4,5) that gives a more detailed classification of reversed-phase columns, have been proposed but to this day the "L" classification is still in widespread use. Thus, some chromatographers who do not really understand the issues still believe that "all C18 columns are the same." Simple examples that this is not the case are shown in Figures 1 and 2. In Figure 1, four different C18 silica bonded phase columns are shown for the same separation under the same operating conditions; each phase provides a different chromatogram. To demonstrate that the L system also doesn't hold for other bonded phases as well, Figure 2 provides an example of three different C8 (L7) columns, one of which (Figure 2[b]) was very similar to the original chromatogram and could probably be substituted in an HPLC method while the third column (Figure 2[c]) is quite different and might even be considered as orthogonal to the first two columns. The Fs designation shown alongside each chromatogram is a numerical classification of how "close" of a fit columns are to one another (4,5). Close Fs numbers are potentially replacement columns while large values of Fs imply that the column would not be a "drop-in" replacement in a particular HPLC method and, in fact, might be a useful column when first performing method development because it offers a different selectivity to the other columns. So, the bottom line is: All C18 (L1) and other reversed phase columns are not the same.

Figure 2: Separation of the same mixture on three reversed-phase columns under the same conditions: (a) Ace C8 (Advanced Chemical Technologies); (b) Precision C8 (Mac-Mod); (c) Inertsil C8 (GL Sciences). Column dimensions: 15 cm à 4.6 mm; flow rate: 2.0 mL/min; temperature: 35 °C; mobile phase: 50:50 30 mM potassium phosphate buffer (pH 2.8)âacetonitrile. Peaks: 1 = N,N-diethylacetamide, 2 = nortriptyline, 3 = 5,5-diphenylhydantoin, 4 = benzonitrile, 5 = anisole, 6 = toluene, 7 = cis-chalcone, 8 = trans-chalcone, 9 = mefenamic acid. (Courtesy of Lloyd Snyder and John Dolan, LC Resources).
Myth 8: Never Use 100% Water with a Reversed-Phase LC Column
False: This myth was brought about by users who experienced a phenomenon popularly known as "phase collapse" when using reversed-phase columns with a low percentage of organic solvent or 100% water as a mobile phase. Phase collapse really is a misnomer as the phenomenon was better explained as phase dewetting. Phase dewetting is highly undesirable since retention times decrease and are not reproducible, peaks may become distorted and reequilibration times may be quite long. Earlier, we published two detailed papers on this subject (6,7). The phase dewetting conditions most often occur when users are trying to increase the retention of very polar compounds in reversed-phase LC by decreasing the percentage of organic solvent in the mobile phase to low values to increase the retention of these polar compounds, which have a tendency to be eluted very early in the chromatogram. Nowadays, this problem is frequently addressed by using hydrophilic interaction liquid chromatography (HILIC).
With the help of Figure 3, I will try to explain phase dewetting. Figure 3 shows two situations: Situation A, where the aqueous mobile phase has a significant portion of water-soluble organic solvent, such as methanol or acetonitrile — a densely chemically bonded C18 (or other hydrophobic bonded phase) prefers to be solvated with organic solvent (for example, like-like relationship); and situation B, when the mobile phase has a very low percentage of organic content (<10%) or even 100% water. A very simplistic visualization of phase collapse can be observed in the upper portion of Figure 3. Situation A shows the C18 bonded group being solvated with methanol and in this state the hydrophobic moieties are able to interact with the hydrophobic portions of solute molecules and provide retention. On the other hand, for the right hand side of the upper portion of Figure 3, situation B shows a C18 phase in a 100% water mobile phase. The C18 functionality prefers to be in a self-associated state (like prefers like) and folds upon itself in a collapsed state. The bottom portion of Figure 3 shows the situation as it actually happens. Most of the interactions with an LC stationary phase occur inside the pores (rather than on the outer surface), so when an organic solvent is present at higher concentrations (greater than 10%), the pores are filled with the water–organic mixture that allows the C18 bonded groups to be solvated, and everything behaves normally. However, when the solvent within a pore becomes unfriendly (for example, very low %B or 100% A), there is a tendency to force the water out of the pore, which results in a dewetting phenomenon. This dewetting doesn't occur instantaneously, but can happen over a number of column volumes as the organic solvent is leached out of the solvated bonded phase. As this is happening, the retention of organic solutes may decrease with time and retention times also decrease accordingly. Selectivity can change during this time and peak shapes can become distorted.
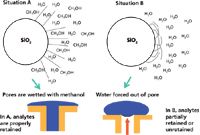
Figure 3: Phase collapse (or more correctly, phase dewetting).
Phase dewetting most often occurs with very hydrophobic, very dense chemically bonded phases. Phases that are highly endcapped with non-polar silane reagents may encourage the situation. The % organic in which dewetting may occur varies with a number of parameters including type of bonded phase, bonded phase coverage (density), pore size, and the presence and availability of residual surface silanol groups among others. Phase dewetting does not permanently damage the column and it can be recovered as described below. However, over the years many chromatographers have been totally baffled by the presence of phase dewetting and much time has been lost trying to solve the problem of shifting retention times. Hence, they believe that one shouldn't run reversed-phase columns in highly aqueous media.
There are two approaches to overcome the phase dewetting phenomenon: Subject the column to a high back pressure according to the Laplace-Young equation (see reference 6 for an example of this approach); or resolvate the stationary phase with a higher % organic in an organic–water mixture or mobile phase.
The first approach is inconvenient and requires a lot of experiments to get the right back pressure. The second approach is the easiest to perform because the column is already installed in the instrument and the experimental conditions can be adjusted to ensure that sufficient organic solvent is present to resolvate the phase. Of course, a third approach is to use a phase that is solvated under all mobile phase conditions (see below).
To illustrate what can happen in a phase dewetting situation, Figure 4 provides an example of the separation of procainamides on a very hydrophobic-C8 phase. The sequence of the experiments is outlined in the figure caption and will not be repeated here. Figure 4(a) depicts the normal isocratic separation that occurs after the stationary phase solvated with an acetonitrile-buffered water mixture. Then, the mobile phase was 90% ammonium dihydrogen phosphate buffer and 10% acetonitrile, which allowed solvation of the hydrophobic stationary phase. Next, the column was rinsed for a period of time with an aqueous buffer (no organic). The mobile phase was returned to the original conditions and the sample was reinjected. Note the greatly reduced elution times and the change in selectivity that occurred with the sample chromatogram (Figure 4[b]). Clearly, this separation is different from what would be expected. The water rinse caused a change in the retention characteristics of the stationary phase most likely by a phase dewetting phenomenon. Next, the column was treated with a 50:50 mixture of aqueous buffer and acetonitrile, followed by the mobile phase. Indeed, the chromatogram returned to the original one shown in Figure 4(a).

Figure 4: Inconsistent retention in a highly aqueous mobile phase as demonstrated by the separation of procainamides on a hydrophobic C18 column. Sequence of events: Condition with a 50:50 mixture of phosphate buffer and acetonitrile for 15 min; run mobile phase for 5 min; inject the sample and obtain the chromatogram shown in (a); switch to 100% aqueous for 30 min; switch back to mobile phase for 5 min; inject the sample and obtain the chromatogram shown in (b); repeat the first three steps; chromatogram returns to (a). Column: 150 mm à 4.6 mm Eclipse XDB-C8; mobile phase: 90% 50 mM KH2PO4 (pH 3.5), 10% acetonitrile; flow rate: 1.0 mL/min; temperature: room temperature. Peaks: 1 = uracil, 2 = procainamide, 3 = N-acetylprocainamide, 4 = N-propionylprocainamide, 5 = caffeine.
Table 1 provides a list of phases that do not show the phase dewetting phenomenon. These phases all have a polar functional group of some kind close to the surface, near or on the chemically bonded phase. Polar embedded phases are among the most popular of these special phases. In this case, a polar functional group is located on the alkyl phase itself usually only a few carbon atoms removed from the silica surface. Different commercial phases utilize different embedded functional groups, the most popular being amide, urea, and carbamate. With these polar groups, the water in the mobile phase can interact and solvate the phase so that collapse or dewetting doesn't occur. Some columns have incorporated polar functional groups in other ways such as endcapping with a polar functionality (for example, diol). These phases are usually given an AQ designation. Very short chain phases (such as C2) do not show phase dewetting because they may allow residual silanol groups to hydrogen bond with the aqueous component of the mobile phase. Surprisingly, very long alkyl chain phases do not show phase dewetting, most likely because the steric requirement allow surface silanols to remain on the bonded silica; hence, water can interact with these silanols and allow surface solvation. Phases with wide pore diameters (for example, 300 Å) do not show phase dewetting because the pores are wide enough not to force water out of them, although I am not aware of any studies on the wettability of these wide-pore phases.
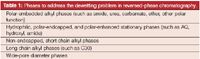
Table 1: Phases to address the dewetting problem in reversed-phase chromatography.
Figure 5 shows the use of an AQ-type phase in a situation with a low percentage of organic mobile-phase content. The sequence of experiments with this column was very similar to that shown in Figure 4. Because this phase was developed to work in mobile phases with a low percentage of organic content and 100% water, it did not undergo phase dewetting when subjected to the same conditions of the highly hydrophobic phase of Figure 4. Such phases are to be recommended for the separation of small polar compounds that are lowly retained on many reversed-phase chromatography columns.

Figure 5: Consistent retention of procainamides. Sequence of events: condition with a 50:50 mixture of phosphate buffer and acetonitrile for 15 min; run mobile phase for 5 min; inject the sample and obtain the chromatogram shown in (a); switch to 100% aqueous for 30 min; switch back to mobile phase for 2 min; inject the sample and obtain the chromatogram shown in (b). Column: 150 mm à4.6 mm, 5-µm dp Zorbax SB-Aq; mobile phase: 90% 50 mM KH2PO4 (pH 3.5), 10% acetonitrile; flow rate: 1.0 mL/min; temperature: room temperature. Peaks: 1 = uracil, 2 = procainamide, 3  = N-acetylprocainamide, 4 = N-propionylprocainamide, 5 = caffeine.
Myth 7: It Takes a Minimum of 10 Column Volumes to Reequilibrate an LC Column
False: Equilibration time is very important in gradient chromatography because it is a limiting factor in the throughput of the technique. At the conclusion of gradient, the column must be returned to its original state before another injection can be made. The longer it takes for this reinstatement to occur, the longer the overall gradient run. In addition, if one takes longer to reequilibrate the column than is actually required, solvent is wasted. In modern two-dimensional liquid chromatography (2D LC×LC), the throughput is dictated by the speed of the secondary column because the flow on the primary column is not stopped during the second chromatographic step. If 10 column volumes are required instead of just a few, then the comprehensive chromatography, already a fairly slow process, is made even slower. Finally, if reequilibration time is too short and the column has not been stabilized, then repeatability of retention time, important when this parameter is used to help identify components, may be limited.
There have been a number of studies of the reequilibration times, but for the purposes of brevity, I would like to cite two of the more comprehensive ones (8,9). Schellinger, Stoll, and Carr (8) studied high-speed gradient elution in the reversed-phase LC of neutral compounds and bases in buffered eluents with regard to retention repeatability and column reequilibration and conducted a follow-up study of full equilibrium conditions (9). There are many variables affecting the reequilibration in reversed-phase LC. In isocratic LC, there is no equilibration time at the conclusion of a chromatographic run, but there may be a considerable waiting time when changing solvent composition. In gradient LC, the eluent composition, the bonding density of the reversed-phase LC packing, the instrumental design (particularly, the gradient delay volume also known as the dwell volume), the flow rate, the use or lack of use of bonded phase additives to wet the bonded phase, and the types of solutes (ionic, ionizable, neutral) and flushing times all play a part.
I would like to summarize the major outcomes of these reequilibration studies. First, the instrument gradient delay volume, although important in the real world, must be subtracted from the total volume of the eluent passed through the column because the delay volume itself has little to do with the reequilibration time that actually occurs within the chromatography column. Earlier liquid chromatographs often had several millilitres of delay volume. The delay volume is the total volume from the point of solvent mixing to the head of the column. There are many volumes to flush out before the actual gradient reaches the column. For high-pressure mixing, the volume of the mixing tee, mixer (if present), pulse damper, pressure transducer, all the connecting tubings, injector including the loop or by-pass volume, and guard column can be substantial. For low-pressure gradient systems, the proportioning valve, inlet and outlet check valves, the pump piston chamber, and various tubing adds even more to the gradient delay volume. More recently, newer UHPLC instruments have addressed these problems by greatly decreasing the instrumental contributions to gradient delay volume as well as extracolumn volumes.
The reequilibration study came up with several conclusions. There are two types of equilibrium: Repeatable equilibrium and full equilibrium. Repeatable equilibrium means that full equilibrium may not have been achieved, but on a practical basis, if the retention time repeatability on subsequent runs is less than 0.002 min then for non-ionizable solutes in unbuffered eluents and for basic compounds using the popular trifluoroactic acid and formic acid additives, repeatable equilibrium can be achieved within two column volumes. For a non-endcapped phase, 1% (v/v) n-butanol added to the mobile phase was required to achieve rapid full equilibrium in two column volumes. For an endcapped phase, the n-butanol was not required.
Myth 6: Superficially Porous (Solid-Core) Particles Have a Significantly Lower Sample Loading Capacity Compared to Totally Porous Particles
False: The sample capacity of an HPLC packing material is proportional to the available surface area, which, of course, is related to the amount of bonded phase chemically attached to the available silanols through monomeric bonding. If one goes through the mathematical calculations of the volume of a 2.7-µm spherical totally porous particle (TPP) and compares the volume of a 2.7-µm superficially porous packing (SPP) with a 0.5-µm porous shell, the total volume available on the SPP particle is about 25% less than the TPP. This assumes that the porous portion of both particles has the same characteristics, which may not be the case. Nevertheless, the loss in surface volume of the SPP is nowhere near that of the pellicular packings of yesteryear in which the shell thickness was 1–2 µm and the particle size was 45–50 µm. If the surface area of the SPP is actually larger than that of the TPP, then the difference in sample capacity between the two could be less (this may be the case).
Rather than relying on mathematical estimates, experiments were actually performed on comparing the TPP and SPP with similar chromatographic conditions for basic compounds. For the basic compound dextromethorphan, successively larger concentrations were injected onto the four columns. Three of the columns were SPP columns Poroshell 120 EC-C18 (100 mm × 3.0 mm, 2.7-µm dp, Agilent Technologies); Ascentis Express C18 (100 mm × 3.0 mm, 2.7-µm dp, Sigma Aldrich/Supelco); and Kinetex C18 (100 mm × 4.6 mm, 2.6-µm dp, Phenomenex). The TPP column was the Zorbax Eclipse Plus C18 (100 × 3.0 mm, 1.8-µm dp, Agilent Technologies). At the time, only a 100 mm × 4.6 mm Kinetex column was available, so adjustments were made in the experimental conditions to accommodate the different column dimensions.

Figure 6: Sample loading of a basic compound (dextromethorphan) onto superficially porous and sub-2-µm totally porous columns. A 10% increase in peak width for the superficially porous particle columns occurs roughly at the same loading as the 1.8-µm totally porous particle column. Mobile phase: 80% 25 mM Na2HPO4 buffer (pH 3.0), 20% acetonitrile; detection: UV absorbance at 205 nm; temperature: 30 °C. Columns: blue diamonds: 100 mm à 3.0 mm, 2.7-µm dp Agilent Poroshell 120 EC-C18; orange squares: 100 mm à 3.0 mm, 2.7-µm dp Supelco Ascentis Express C18; green triangles: 100 mm à 4.6 mm, 2.6-µm dp Phenomenex Kinetex C18; yellow X’s: 100 mm à 3.0 mm, 1.8-µm dp Agilent Zorbax Eclipse Plus C18.
One definition of overload is when the sample size injected causes a 10% dropoff in efficiency (or alternatively a 10% increase in peak width). One can view the results of the experiment in Figure 6, which shows a plot of the peak width versus sample concentration injected at a constant volume. A 10% increase in peak width for the SPP columns occurred at roughly the same sample loading as for the totally porous column at a concentration value of 0.05 mg/mL indicating a comparable sample capacity for both types of columns. Interestingly, a recent similar study by Fallas and colleagues (10) came to the same conclusion. Table 2 is an abbreviated extract of data from their work, which shows that, for both basic and acidic compounds on the Poroshell 120 EC-C18 and the totally porous Zorbax Eclipse Plus C18 columns of the same dimensions and same conditions used, has nearly the same sample capacity. Interestingly, other SPP columns and porous particle columns were evaluated in the same study. All of them weren't very different, indicating the sample capacity of a number of porous and SPPs when tested under the same conditions were essentially the same. However, with some of the newer SPPs with a thinner shell thickness, there may be different sample capacities than TPPs of the same size.

Table 2: Sample capacity for two C18 columns.
"Column Watch" Editor Ronald E. Majors is a senior scientist at the Columns and Supplies Division, Agilent Technologies, Wilmington, Delaware, USA, and is a member of the LCGC Asia Pacific editorial advisory board. Direct correspondence about this column should be addressed to "Column Watch", LCGC Asia Pacific, 4A Bridgegate Pavilion, Chester Business Park, Wrexham Road, Chester, CH4 9QH, UK, or e-mail the editor-in-chief, Alasdair Matheson, at amatheson@advanstar.com
References
(1) R.E. Majors, LCGC North Am. 24(11), 1172–1182 (2006).
(2) R.E. Majors, "Top Ten LC Column Myths, Lecture PL2" presented at HPLC 2013, Amsterdam, Amsterdam, The Netherlands, 2013.
(3) R.E. Majors, LCGC North Am. 25(1), 31–39 (2012).
(4) L.R. Snyder and J.W. Dolan, LCGC North Am. 22(12), 1146–1152 (2004).
(5) L.R. Snyder and J.W. Dolan, LCGC North Am. 23(2), 118–127 (2005).
(6) M. Przybyciel and R.E. Majors, LCGC North Am. 20(6) 516–523 (2002).
(7) R.E. Majors and M. Przybyciel, LCGC North Am. 20(7), 584–593 (2002).
(8) A.P. Schellinger, D.R. Stoll, and P.W. Carr, J. Chromatogr. A 1192(1), 41–53 (2008).
(9) A.P. Schellinger, D.R. Stoll, and P.W. Carr, J. Chromatogr. A 1192(1), 54–61 (2008).
(10) M.M. Fallas, S.M.C. Buckenmaier, and D.V. McCalley, J. Chromatogr. A 1235, 49–59 (2012).
New Study Uses MSPE with GC–MS to Analyze PFCAs in Water
January 20th 2025Scientists from the China University of Sciences combined magnetic solid-phase extraction (MSPE) with gas chromatography–mass spectrometry (GC–MS) to analyze perfluoro carboxylic acids (PFCAs) in different water environments.
The Next Frontier for Mass Spectrometry: Maximizing Ion Utilization
January 20th 2025In this podcast, Daniel DeBord, CTO of MOBILion Systems, describes a new high resolution mass spectrometry approach that promises to increase speed and sensitivity in omics applications. MOBILion recently introduced the PAMAF mode of operation, which stands for parallel accumulation with mobility aligned fragmentation. It substantially increases the fraction of ion used for mass spectrometry analysis by replacing the functionality of the quadrupole with high resolution ion mobility. Listen to learn more about this exciting new development.
A Guide To Finding the Ideal Syringe and Needle
January 20th 2025Hamilton has produced a series of reference guides to assist science professionals in finding the best-suited products and configurations for their applications. The Syringe and Needle Reference Guide provides detailed information on Hamilton Company’s full portfolio of syringes and needles. Everything from cleaning and preventative maintenance to individual part numbers are available for review. It also includes selection charts to help you choose between syringe terminations like cemented needles and luer tips.
The Complexity of Oligonucleotide Separations
January 9th 2025Peter Pellegrinelli, Applications Specialist at Advanced Materials Technology (AMT) explains the complexity of oligonucleotide separations due to the unique chemical properties of these molecules. Issues such as varying length, sequence complexity, and hydrophilic-hydrophobic characteristics make efficient separations difficult. Separation scientists are addressing these challenges by modifying mobile phase compositions, using varying ion-pairing reagents, and exploring alternative separation modes like HILIC and ion-exchange chromatography. Due to these complexities, AMT has introduced the HALO® OLIGO column, which offers high-resolution, fast separations through its innovative Fused-Core® technology and high pH stability. Alongside explaining the new column, Peter looks to the future of these separations and what is next to come.
Oasis or Sand Dune? Isolation of Psychedelic Compounds
January 20th 2025Magic mushrooms, once taboo, have recently experienced a renaissance. This new awakening is partially due to new findings that indicate the effects of psilocybin, and its dephosphorylated cousin psilocin may produce long lasting results for patients who might be struggling with anxiety, depression, alcohol and drug abuse, and post-traumatic stress disorder. Hamilton Company has developed a methodology for the isolation and identification of 5 common psychedelic compounds used in the potential treatment of disease. The PRP-1 HPLC column resin remains stable in the harsh alkaline conditions ideal for better separations.
