Screening of Drugs of Abuse Using the Velox 360 Paper Spray System
The Application Notebook
Paper spray mass spectrometry (MS) allows for the direct analysis of pharmaceuticals, drugs of abuse, and other small molecules from blood, urine, and other biofluids (1,2).
Paper spray mass spectrometry (MS) allows for the direct analysis of pharmaceuticals, drugs of abuse, and other small molecules from blood, urine, and other biofluids (1,2). Described herein is the application of a Velox 360 paper spray autosampler and ion source for the direct analysis of drugs of abuse in urine samples.
Paper spray is performed by depositing the sample directly onto a porous cellulosic substrate contained within a disposable cartridge (Velox Sample Cartridge). The sample is allowed to dry, and the cartridge is inserted into the automated ion source for analysis. The Velox 360 autosampler and ion source performs all of the steps necessary to perform the analysis, including loading the cartridge, depositing extraction solvent onto the cartridge, positioning the cartridge in front of the mass spectrometer inlet, and applying the ionization voltage. Ions are generated from the sharp tip of the porous substrate contained within the cartridge. The Velox 360 paper spray system provides an automated, cost effective, and efficient method for screening DOAs using mass spectrometry with minimal sample preparation.
This application note describes the use of the Velox 360 System and high resolution mass spectrometry for rapid screening of illicit drugs and pharmaceuticals from urine samples
Experimental
Anonymous urine samples from a methadone clinic were obtained from Clinitox Dx (Ontario, Canada). First, 5 µLs of urine were deposited onto the Velox sample cartridge using a pipette. The sample was allowed to dry for 30 min at room temperature or was dried in a 40° incubator for 10 min. The cartridges were then analyzed automatically using the Velox 360 coupled to a Thermo Scientific Exactive™.
Results
A typical mass spectrum from a urine sample collected at a methadone clinic is shown in Figure 1. The intense signals from various drugs of abuse, along with creatine, are shown in the mass spectrum and are within 3 ppm of the theoretical mass-to-charge ratio. The presence of these drugs in the urine sample was further confirmed by HPLC–MS-MS (data not shown).
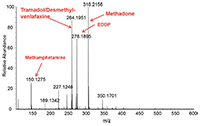
Figure 1: Full mass spectrum recorded using paper spray directly from a dried urine sample showing the presence of various substances including the (M+H+) ions of: creatine (m/z 114.066), methamphetamine (m/z 150.158), tramadol/desmethyl-venlafaxine (m/z 264.195), EDDP (m/z 278.189), and methadone (m/z 310.215).
Conclusion
This application note demonstrates the suitability of paper spray mass spectrometry for the rapid screening of illicit drugs and drug metabolites in urine, particular when used in combination with high resolution MS instrumentation. Compared to more traditional approaches, the Velox 360 offers several compelling advantages for this application, including:
1) No sample preparation. Analysis is done directly from urine.
2) Rapid analysis. Less than 2 min per sample for the entire analytical procedure (excepting time required to dry samples).
3) No carry-over. Because the analysis is done from a single use cartridge, there is no worry that high concentration samples will cause carry-over.
4) No chromatography. That means no columns to replace, no carry-over on the column, and no chromatography methods to develop.
References
(1) H. Wang, J. Liu., R.G. Cooks, and Z. Ouyang, Angew. Chem., Int. Ed. 49, 877–880 (2010).
(2) N.E. Manicke, Q.A. Yang, H. Wang, S. Oradu, Z. Ouyang, and R.G. Cooks, Int. J. Mass Spectrom. 300, 123–129 (2011).

Prosolia Inc.
6500 Technology Center Drive, Suite 200, Indianapolis, IN 46278
tel. +1(866) 241-0239, fax +1(317) 873-3175
Website: www.prosolia.com

A Novel LC–QTOF-MS DIA Method for Pesticide Quantification and Screening in Agricultural Waters
May 8th 2025Scientists from the University of Santiago de Compostela developed a liquid chromatography quadrupole time-of-flight mass spectrometry (LC–QTOF-MS) operated in data-independent acquisition (DIA) mode for pesticide quantification in agriculturally impacted waters.
Investigating 3D-Printable Stationary Phases in Liquid Chromatography
May 7th 20253D printing technology has potential in chromatography, but a major challenge is developing materials with both high porosity and robust mechanical properties. Recently, scientists compared the separation performances of eight different 3D printable stationary phases.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)


















