Analysis of Monoclonal Antibody Aggregates by SEC Using MS-Friendly Mobile Phases
The use of mass spectrometry as a means of detection is becoming increasingly more common among research laboratories in the field of proteomics.
The use of mass spectrometry as a means of detection is becoming increasingly more common among research laboratories in the field of proteomics. After more than 15 years of research, LC–MS systems are now more robust, and used more often for routine analyses which are nearly unachievable by any other mode of detection.
Separation of protein aggregates from their native species is often performed using size exclusion chromatography (SEC), as this mode allows for the analysis of various components in a sample on the basis of their hydrodynamic radius in solution. Conventional SEC separations make use of relatively high ionic strength mobile phase compositions in an effort to minimize ionic interactions between the analyte and stationary phase. Due to the substantial amount of salt present in the mobile phase, on-line interfacing with mass spectrometry is not feasible due to the inevitable contamination of the MS ion source by the mobile phase salts. In order to make SEC-MS an applicable technique, volatile, MS-friendly mobile phase compositions must be implemented to avoid damage to the MS system. The challenge of using such mobile phase compositions exists due to the absence of salts which hinder ionic interactions during a separation.
This application note illustrates the effective use of MS-friendly mobile phase compositions in the analysis of monoclonal antibody aggregates using a TSKgel UltraSW Aggregate SEC column. The TSKgel® UltraSW Aggregate column demonstrates high stability and low reactivity via ionic interactions even in low salt and no salt environments most likely due to the diol coating of the silica stationary phase.
Materials and Methods
Instrumentation: Agilent 1200 HPLC system run by Chemstation® (ver. B.04.02)
Column: TSKgel UltraSW Aggregate, 3 µm, 7.8 mm ID × 30 cm
Mobile phase: 100 mmol/L PO4/100 mmol/L SO4, pH 6.7 20% CH3CN/0.1% TFA/0.1% FA 100 mmol/L ammonium bicarbonate, pH 7.0
Gradient: Isocratic
Flow rate: 1 mL/min
Detection: UV @ 280 nm
Temperature: 25 °C
Injection vol.: 10 µL
Sample: TBL mAb 1 (4.0 mg/mL)
Results and Discussion
Figure 1 illustrates the separation of mAb 1 using three different mobile phase compositions. All three mobile phase compositions yielded highly similar results for all peak parameters of the mAb 1 monomer. Additionally, slightly later elution of the mAb 1 monomer was observed when separated using the salt-based mobile phase. The observed shift in retention time of the mAb 1 monomer peak represents a %RSD of 0.1% among the three mobile phase conditions. Similarly average peak area of the mAb 1 monomer was found to be highly reproducible as well, yielding a %RSD of 0.53% across the three mobile phase systems evaluated in this work. This illustrates that the observed differences in retention time and peak areas obtained from the three mobile phase systems are not statistically significant.
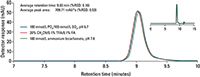
Figure 1: Separation of mAb 1 using volatile and salt-based mobile phase compositions on the TSKgel UltraSW Aggregate column.
As characterization of protein aggregates is of increasing importance in research, and with significant work being directed towards analysis by MS, the mAb 1 antibody was subjected to thermal stress for forced aggregation to evaluate the various mobile phase systems in this context. As shown in Figure 2, aggregates of mAb 1 are clearly separated from the monomeric species using all three mobile phase compositions. Similar to Figure 1, results for critical peak parameters of the mAb 1 monomer are highly reproducible regardless of the mobile phase composition. Additionally, the total peak area (monomer and aggregate) obtained using the three mobile phase systems was highly similar to one another (avg: 671.04 mAU*s), and yielded a %RSD of 3.54%, illustrating the differences are not statistically significant. It is also of note that the observed shift in retention time of the mAb 1 monomer peak only corresponds to a %RSD of 0.12% for all three mobile phase compositions.
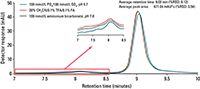
Figure 2: Separation of forced aggregated mAb 1 using volatile and salt-based mobile phase compositions on the TSKgel UltraSW ggregate column.
Conclusions
The growing interest in both protein aggregate analysis and mass spectrometry in the field of proteomics demand effective SEC-MS methods utilizing suitable mobile phases. The use of volatile mobile phase systems, such as 20% acetonitrile, 0.1% trifluoroacetic acid, and 0.1% formic acid or 100 mmol/L ammonium bicarbonate at pH 7.0 yield highly reproducible separation of mAb 1 aggregates, with similar or better performance over traditional salt-based mobile phase compositions. These results also show the effectiveness of the diol coating on the TSKgel UltraSW Aggregate column to assist in minimizing ionic interaction which are frequently present between the sample and stationary phase in low salt environments.
Tosoh Bioscience and TSKgel are registered trademarks of Tosoh Corporation.
Chemstation is a registered trademark of Agilent Technologies Inc.

Tosoh Bioscience LLC
3604 Horizon Drive, Suite 100, King of Prussia, PA 19406
tel. (484) 805-1219, fax (610) 272-3028
Website: www.tosohbioscience.com

SEC-MALS of Antibody Therapeutics—A Robust Method for In-Depth Sample Characterization
June 1st 2022Monoclonal antibodies (mAbs) are effective therapeutics for cancers, auto-immune diseases, viral infections, and other diseases. Recent developments in antibody therapeutics aim to add more specific binding regions (bi- and multi-specificity) to increase their effectiveness and/or to downsize the molecule to the specific binding regions (for example, scFv or Fab fragment) to achieve better penetration of the tissue. As the molecule gets more complex, the possible high and low molecular weight (H/LMW) impurities become more complex, too. In order to accurately analyze the various species, more advanced detection than ultraviolet (UV) is required to characterize a mAb sample.















