Characterization of Amyloid Fibrils Formed by the Cell Death Regulator Bcl-2
Proteins of the Bcl-2 family are molecular transducers sensitive to internal and external apoptotic signals that play a key role in the regulation of apoptosis.
Proteins of the Bcl-2 family are molecular transducers sensitive to internal and external apoptotic signals that play a key role in the regulation of apoptosis. Their aggregation can lead to the formation of amyloid fibers due to protein misfolding which are associated with numerous diseases.
The work described in this application note focused on the in vitro formation of aggregates by a Bcl-2 protein initiated by incubation of the protein at 37 °C. Multi-detection size exclusion chromatography (SEC) was used to characterize the early events occurring during the aggregation process following incubation for 1 day and 1 week. SEC was performed using a Superose 6HR (GE Healthcare) with a buffer of 20 mM sodium phosphate, pH 8, and 150 mM sodium chloride. The Viscotek TDA with UV, RI, light scattering, and viscometer detectors was used to determine the molecular weight (MW) and intrinsic viscosity (IV).
Results and Discussion
Figure 1 is a typical chromatogram following 1 day of incubation at 37 °C. Three main species were detected, with molecular weights of 74 kDa, 51 kDa, and 25 kDa. The main population of this sample was found to be the protein's monomer. An additional species was detected by the light scattering detector eluting at 11 mL, which is a high molecular weight aggregate but at very low concentration.
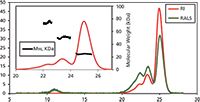
Figure 1: Chromatogram of Bcl-2 from the Viscotek TDAmax. RALS (green line) and refractive index detector (red line). Inset shows the molecular weight distribution of the different species within the sample.
The samples were also analyzed after incubation for 1 week at 37 °C. Following this extended incubation period more aggregates were found to have formed. The SEC experiments showed that the first step of the fiber growth is the formation of small aggregates, mostly dimers and trimers, which further assemble into larger aggregates. This information was only possible to obtain with the use of multi-detector SEC using the TDA system.
Work performed in conjunction with the Institut Pasteur, Paris, France
References
(1) A. Chenal, C. Vendrely, H. Vitrac, J.C. Karst, A. Gonneaud, C.E. Blanchet, S., Pichard, E. Garcia, B. Salin, P. Catty, D. Gillet, N. Hussy, C. Marquette, C. Almunia, and V. Forge, "Amyloid Fibrils Formed by the Programmed Cell Death Regulator Bcl-xL.," J. Mol. Biol. 415, 584–599 (2012).
(2) J.C. Karst, A.C. Sotomayor-Pérez D. Ladant, and A. Chenal, "Estimation of Intrinsically Disordered Protein Shape and Time-Averaged Apparent Hydration in Native Conditions by a Combination of Hydrodynamic Methods," Methods Mol. Biol. 896, 163–77 (2012).

Malvern Instruments Ltd.
Enigma Business Park, Groveland Road, Malvern, UK
tel. +44 (0) 1684 892456, Email: salesinfo@malvern.com
Website: www.malvern.com

SEC-MALS of Antibody Therapeutics—A Robust Method for In-Depth Sample Characterization
June 1st 2022Monoclonal antibodies (mAbs) are effective therapeutics for cancers, auto-immune diseases, viral infections, and other diseases. Recent developments in antibody therapeutics aim to add more specific binding regions (bi- and multi-specificity) to increase their effectiveness and/or to downsize the molecule to the specific binding regions (for example, scFv or Fab fragment) to achieve better penetration of the tissue. As the molecule gets more complex, the possible high and low molecular weight (H/LMW) impurities become more complex, too. In order to accurately analyze the various species, more advanced detection than ultraviolet (UV) is required to characterize a mAb sample.















