New Solution for Oligonucleotides Separation Using Scherzo SW-C18 Column
Oligonucleotides are in the center of many recent research projects including genetic testing, polymerase chain reactions (PCR), forensics, and DNA/RNA silencing.
Oligonucleotides are in the center of many recent research projects including genetic testing, polymerase chain reactions (PCR), forensics, and DNA/RNA silencing. Two major challenges for large-scale applications of oligonucleotides in these fields include determination of molecular weight of the individual chains and the detail of the base sequence as in the case where single base changes could have major implications in function.
Current common methods for oligonucleotides separations typically include ion-pair reagents in reversed phase chromatography or anion-exchange HPLC. Both of these strategies are not ideal for chromatographers because they often suffer from poor reproducibility, and short column life times. These strategies also add the cost of additional reagents as in the case of ion-pairing, and often require high pHs which cause rapid deterioration of silica-based columns. Furthermore, these techniques are typically not sufficiently selective to separate oligonucleotide isomers.
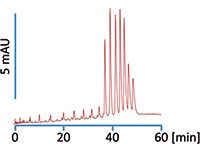
Figure 1: Efficient separation of oligonucleotides using Scherzo SW-C18.
In this article, we show the successful application of Scherzo SW-C18 column for oligonucleotide separation, addressing the two main analytical goals of chromatographers in these fields — chain size discrimination and base pair substituted isomer separation. The Scherzo SW-C18 is a unique multi-mode column that contains reversed phase ODS ligands, as well as both cation and anion exchange (Figure 2). This places Scherzo SW-C18 at a distinct advantage in the analysis of oligonucleotides because you get the selective retention characteristics required for this difficult separation without the need of an ion-pairing reagent. Also, since this column has built-in ion exchange sites, you can have the advantage of IEX with the ease and reliability of reverse phase mobile phase conditions.
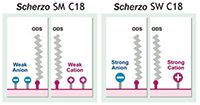
Figure 2: Schematic comparison of the unique stationary phase composition of Scherzo SW-C18 and Scherzo SM-C18.
Experimental
A Scherzo SW-C18, 150 × 3 mm column was used. Elution conditions were: (A) 10 mM ammonium acetate, (B) 200 mM ammonium acetate/acetonitrile (85/15), 40–70% B in 0–65 min, injection volume 1 µL (1 µg), 1 mL/min flow rate, 20 MPa, and 50 °C column temperature. Eluent was analyzed using UV detector at 260 nm.
Discussion
Figure 1 shows separation of oligothymidylic acid ammonium salt with 12 to 18 mers. A very good speciation is observed based only on a single mer difference in oligonucleotides' size and mass. It should be noted the separation presented in Figure 1 was acquired without using ion-pairing reagents or high pH regime. That indicates not only immediate savings in time and resources in lab, but also increased column lifetime.
The ideal column for oligonucleotide separation would provide not only excellent separation based on number of mers, but also would exhibit superior selectivity for isomers. As seen in Figure 1, our Scherzo SW-C18 column shows excellent size resolution, however in a recent work described in detail elsewhere, it was shown that a sister column to the SW-C18, the Scherzo SM-C18, can separate isomers with structural changes as small as a single base for a 21 mer oligonucleotide. Significant structural changes such as N-x deletion are also separated using this column (Biba et al., J. Chromatogr. A1304, 69–77 [2013]).
In summary, Scherzo columns exhibit excellent capabilities for extremely challenging separations of oligonucleotides. Individual species are separated based on their size as well as structural differences; an accomplishment not available by ion-pair reversed-phase LC or strong-anion-exchange LC alone.

Imtakt USA
1104 NW Overton St., Portland OR, 97209
tel. (888) 456-HPLC, (215) 665-8902, fax (501)646-3497
Website: www.imtaktusa.com

SEC-MALS of Antibody Therapeutics—A Robust Method for In-Depth Sample Characterization
June 1st 2022Monoclonal antibodies (mAbs) are effective therapeutics for cancers, auto-immune diseases, viral infections, and other diseases. Recent developments in antibody therapeutics aim to add more specific binding regions (bi- and multi-specificity) to increase their effectiveness and/or to downsize the molecule to the specific binding regions (for example, scFv or Fab fragment) to achieve better penetration of the tissue. As the molecule gets more complex, the possible high and low molecular weight (H/LMW) impurities become more complex, too. In order to accurately analyze the various species, more advanced detection than ultraviolet (UV) is required to characterize a mAb sample.















