Amino Acid Analysis According to European Pharmacopoeia 8.0
The European Pharmacopoeia (Ph. Eur.) defines requirements for the qualitative and quantitative composition of medicines, as well as the tests to be carried out on medicines and on substances and materials used in their production.
The European Pharmacopoeia (Ph. Eur.) defines requirements for the qualitative and quantitative composition of medicines, as well as the tests to be carried out on medicines and on substances and materials used in their production.
It covers active substances, excipients, and preparations of chemical, animal, human or herbal origin, homoeopathic preparations and homoeopathic stocks, antibiotics, as well as dosage forms and containers. It also includes tests on biologicals, blood and plasma derivatives, vaccines, and radiopharmaceutical preparations. The European Pharmacopoeia and its requirements are legally binding in the member states of the European Pharmacopoeia Convention and the European Union.
All manufacturers of medicines or substances for pharmaceutical use therefore must apply the Ph. Eur. quality standards in order to be able to market and use these products in Europe.
Amino acids analysis can be used for:
- Identification tests on biopharmaceutical active ingredients (such as peptides, proteins) by means of amino acids composition analysis;
- Impurities and related substances determination on active pharmaceutical ingredients (APIs) (such as free amino acids) and intermediates;
- Single or total amino acids quantification in drug products, including markers determination in complex matrixes (such as phytopharmaceuticals).
The following Ph. Eur. monographs have already officially introduced the amino acid analysis method with post-column ninhydrin derivatization as the analytical procedure required for the determination of the ninhydrin-positive substances, and additional papers are expected to be published in upcoming months:
Cysteine HCl Monohydrate 01/2014:0895, Isoleucine 07/2013:0770, Leucine 07/2013:0771, Lysine HCl 07/2013:0930, Serine 01/2014:0788, Proline 01/2014:0785, Threonine 01/2014:1049, Valine 01/2014:0796, Arginine 07/2014:0806
Pickering Laboratories, Inc. offers a complete solution for amino acids analysis according to European Pharmacopoeia 8.0. This includes the Pinnacle PCX post-column derivatization instrument, analytical columns and GARDs, buffers and Trione® Ninhydrin reagent. The Pinnacle PCX is capable of performing column temperature gradients that allow easily modified conditions and improved run times and amino acids separations. The methods presented in this application note were optimized to comply with system suitability requirements of Pharmacopoeia 8.0 methods.
Each Pharmacopoeia monograph describes the preparation of the test and reference solutions specific for each amino acid. The solutions are used for calculations of percentage contents, impurity levels as well as parameters of system suitability. Resolution of 1.5 is required between Leucine and Isoleucine peaks.
For all amino acids, except Cysteine, Sodium-based and Lithium-based methods are available. For Cysteine analysis, only Lithium-based methods are suitable. Sodium-based methods have shorter run times and are preferable for all amino acids except Cysteine.
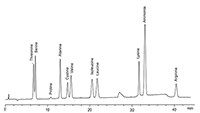
Figure 1: Sodium chromatogram of amino acids analyzed using Pharmacopoeia 8.0 methods (3 µg/mL each, 50 µL injection).
Equipment:
Quaternary HPLC pump, autosampler, UV-vis detector
Pinnacle PCX post-column derivatization system
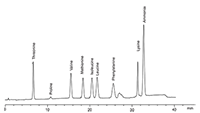
Figure 2: Sodium chromatogram of alternative amino acids analyzed using Pharmacopoeia 8.0 methods (3 µg/mL each, 50 µL injection).
Analytical columns and Eluants:
For Sodium-based methods: High-efficiency Sodium cation-exchange column, 4.6 × 110 mm, Catalog Number 1154110T. Eluants: Na315, Na425, Na640, RG011
For Lithium-based methods: High-efficiency Lithium cation-exchange column, 4.6 × 75 mm, Catalog Number 0354675T. Eluants: 1700-1125, Li365, Li375, RG003
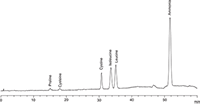
Figure 3: Lithium chromatogram of amino acids used as reference solutions for Cysteine analysis (3 µg/mL each, 50 µL injection).
Post-column reagent
Trione® Ninhydrin Reagent
To make it easier to start using Pickering Laboratories methods, we offer chemistry kits that include: analytical column, GARD, buffers, and reagents for amino acids analysis. All parts of the kit could be ordered individually if needed. Please contact Pickering Laboratories if you have any questions regarding this application.
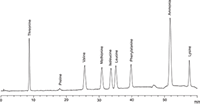
Figure 4: Lithium chromatogram of amino acids analyzed using Pharmacopoeia 8.0 methods (3 µg/mL each, 50 µL injection)
Pickering Laboratories will keep updating its Pharmacopoeia 8.0 methods as new monographs are released. Please contact support@pickeringlabs.com for the latest methods and chromatograms.

Pickering Laboratories, Inc.
1280 Space Park Way, Mountain View, CA 94043
tel. (800) 654-3330, (650) 694-6700
Website: www.pickeringlabs.com

SEC-MALS of Antibody Therapeutics—A Robust Method for In-Depth Sample Characterization
June 1st 2022Monoclonal antibodies (mAbs) are effective therapeutics for cancers, auto-immune diseases, viral infections, and other diseases. Recent developments in antibody therapeutics aim to add more specific binding regions (bi- and multi-specificity) to increase their effectiveness and/or to downsize the molecule to the specific binding regions (for example, scFv or Fab fragment) to achieve better penetration of the tissue. As the molecule gets more complex, the possible high and low molecular weight (H/LMW) impurities become more complex, too. In order to accurately analyze the various species, more advanced detection than ultraviolet (UV) is required to characterize a mAb sample.















