LC–MS-MS Method for the Determination of Enalapril and Enalaprilat from Human Plasma Using SOLA
Thermo Scientific Application Note
A liquid chromatography tandem mass spectrometry method for enalapril and enalaprilat from human plasma has been developed using Thermo Scientific SOLA cartridges. Sample preparation is fast, efficient, and reproducible giving excellent recovery levels for each compound.
SOLA products are a revolutionary new solid phase extraction (SPE) product range. This first-in-class SPE product introduces next-generation, innovative technological advancements, giving unparalleled performance characteristics compared to conventional sample preparation techniques.
SOLA products have significant advantages for the analyst when processing compounds in complex matrices particularly in high throughput bioanalytical and clinical laboratories where reduced failure rate, higher levels of reproducibility, speed of analysis and lower sample/solvent requirements are critical. The increased performance from SOLA products provides higher confidence in analytical results and lowers cost without compromising ease of use or requiring complex method development..
SOLA products ensure good recoveries, accuracy, linearity and precision with a reduction in elution solvent volume, hence reduced solvent costs and subsequently reduced drying times.
Experimental Details
Sample Pretreatment
- Prepare a mixed standard spiking solutions in methanol (enalapril and enalaprilat).
- Prepare a working internal standard solution in water (benazepril).
- Take 200 µL of blank human plasma or sample.
- For standards and quality control (QC) samples add 10 µL of standard spiking solution; for all other samples add 10 µL of methanol.
- For standards, QC's and unknowns add 10 µL of working internal standard solution; for blanks add 10 µL of water.
- Add 4 µL of formic acid.
- Mix well.
Sample Preparation
Compound(s): enalapril, enalaprilat, benazepril (IS)
Matrix: Human plasma
Cartridge type: Thermo Scientific SOLA 10 mg/1 mL p/n 60109-001
Conditioning stage: 1 mL methanol, 1 mL water
Application stage: Load and allow to flow under gravity
Washing stage: 200 µL 0.1% formic acid in water
Elution stage: 2 × 200 µL 2% ammonia solution in methanol
Additional stage: Dry and reconstitute in 200 µL 90:10 (v/v) water/methanol. Sonicate for 5 min.
Separation Conditions
Instrumentation: Thermo Scientific Accela 600
Column: Hypersil GOLD, 1.9 µm, 50 × 2.1 mm p/n 25002-052130
Mobile phase A: water + 0.1% formic acid
Mobile phase B: acetonitrile + 0.1% formic acid
Gradient: 10–100% B in 1 min
Flow rate: 0.6 mL/min
Column temperature: 70 °C
Injection vol: 2.5 µL
Results
Extracted enalapril and enalaprilat standards from human plasma were linear over the dynamic range of 1 and 100 ng/mL with an r2 correlation of 0.9986 and 0.9974. QC samples analysed at a concentration of 50 ng/mL (n=6). Precision and recovery data is shown in Table 1.
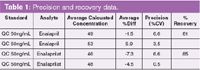
Table 1: Precision and recovery data.
Conclusions
SOLA SPE cartridges and Hypersil GOLD HPLC allow robust and efficient analysis of enalapril and enalaprilat from human plasma to be carried out simply and quickly with excellent reproducibility and recovery being obtained quickly. SOLA provided excellent reproducibility and recovery of the compounds of interest.
Thermo Fisher Scientific
Tudor Road, Manor Park
Runcorn, Cheshire, UK.
Website: www.thermoscientific.com

Separating Impurities from Oligonucleotides Using Supercritical Fluid Chromatography
February 21st 2025Supercritical fluid chromatography (SFC) has been optimized for the analysis of 5-, 10-, 15-, and 18-mer oligonucleotides (ONs) and evaluated for its effectiveness in separating impurities from ONs.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)


















