Separation of Heat-Degraded Recombinant Human EPO Protein
Agilent Application Note
Erythropoietin protein (EPO) is a glycoprotein hormone found in plasma. It is a cytokine for erythrocyte (red blood cell) precursors in the bone marrow. EPO controls red blood cell production, and has neuroprotective activity against a variety of potential brain injuries and antiapoptotic functions in several tissue types. Recombinant human EPO protein (rEPO) is produced by Chinese hamster ovary (CHO) cells using recombinant DNA technology. rEPO is one of the most widely produced proteins worldwide for therapeutic agents.
The HPLC separation of EPO protein from its impurities can be achieved by using a variety of chemistries, including reversed-phase chromatography. In this example, we separated heat-degraded, CHO-derived EPO protein using an Agilent ZORBAX 300SB-C18 1.8 µm column on an Agilent 1290 Infinity LC system.
Experimental
Recombinant human EPO protein was heated at 60 °C overnight (16 h) at neutral pH (pH 7.0) and acidic pH (pH 4.0). Due to its nature, rEPO protein will be degraded or form other isoforms when heated at high temperatures such as 60 °C at different pH. At neutral pH, rEPO forms limited isoforms, but when acidic pH conditions are used, the structure of rEPO protein will be altered significantly. The lower the pH, the greater the change (1).
Column: Agilent ZORBAX RRHD 300SB-C18, 2.1 × 50 mm, 1.8 µm
Sample: Recombinant human EPO protein (rEPO)
Sample conc: 1.0 mg/mL
Injection vol: 3 µL
Flow rate: 1.0 mL/min
Pressure: 650 bar
Mobile phase: A 0.1% TFA in deonized water; B 0.01% TFA in 100% ACN
Gradient: 5–100% B from 0–2.5 min
Detector: UV, 280 nm
System: Agilent 1290 Infinity HPLC
Results and Discussion
Figure 1 shows separations obtained at the same temperature but different pH. Panel (a) shows data from a sample heated at neutral pH. The column resolved the main peak of rEPO from its degraded products or isoforms very well.
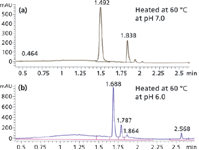
Figure 1: Heat-treated rEPO protein well resolved by the Agilent ZORBAX RRHD 300SB-C18, 2.1 à 50 mm, 1.8 µm column.
Panel (b) shows the separation of heat-treated rEPO at pH 6.0. The separation conditions were the same as for the neutral pH trial but the chromatogram was drastically changed. Heating with acidic conditions can greatly alter and degrade the conformation of the rEPO protein. The retention time of the major peak changed from 1.49 min to 1.69 min and the peak also contained a shoulder peak on the right. In addition, more impurity peaks were also observed. This clearly indicated that the ZORBAX RRHD 300SB-C18, 1.8 µm column could resolve rEPO impurities and degradation products very well, and can be used for monitoring the stability of rEPO.
Reference
(1) Yoshiyuki Endo et al., J. Biochem. 112(5), 700–706 (1992).
Agilent Technologies Inc.
5301 Stevens Creek Blvd., Santa Clara, California, USA
tel: (800) 227-9770 (Directory) fax: (866) 497-1134
Email: info_agilent@agilent.com Website: www.agilent.com/chem/bioHPLCproteins

New Method Explored for the Detection of CECs in Crops Irrigated with Contaminated Water
April 30th 2025This new study presents a validated QuEChERS–LC-MS/MS method for detecting eight persistent, mobile, and toxic substances in escarole, tomatoes, and tomato leaves irrigated with contaminated water.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)


















