A Variety of Agilent ZORBAX RRHD Phases Offers Selectivity Options for the Determination of Anthocyanins in Blueberries with UHPLC/MS
Agilent Application Note
Anne E. Mack, Agilent Technologies, Santa Clara, California, USA.
Blueberries are extracted into acidified methanol and analysed for anthocyanins with a methanol/formic acid gradient via UHPLC/MS; four different Agilent ZORBAX RRHD stationary phases are quickly evaluated. The Phenyl-Hexyl column separates the most anthocyanin peaks from the blueberry sample, while the SB-Phenyl is the most orthogonal of the columns.
Advancements in liquid chromatography have led to significantly improved sample throughput. The Agilent 1290 Infinity UHPLC and Agilent ZORBAX Rapid Resolution High Definition (RRHD) columns are manufactured to withstand pressures up to 1200 bar. This allows the use of faster flow rates and rapid column screening to easily take advantage of stationary phase selectivity differences during method development.
Two different phenyl columns are currently available within the ZORBAX RRHD family, Eclipse Plus Phenyl-Hexyl and StableBond SB-Phenyl. Both phases excel with the analysis of anthocyanins due to the compounds' abundant conjugation. The π electrons in double bonds in these compounds interact with the π electrons in the phenyl stationary phase, providing a unique selectivity mechanism over traditional alkyl phases, such as C18 (3). While the π-π interactions are not as strong as the hydrophobic interactions responsible for retention with alkyl phases, they may provide slight selectivity advantages for phenyl columns when analysing closely related conjugated compounds.
Experimental
An Agilent 1290 Infinity LC System with an Agilent G6410A Triple Quadrupole Mass Spectrometer was used in this experiment.
Mobile Phase: A – 5% formic acid in water
B – methanol
Flow rate: 0.65 mL/min
Gradient: 10–50% B in 15 min
Sample 5 µL injection of blueberry extract:
TCC: 30 °C
MS: MS2 Scan: 200–1000, ESI
positive mode
Scan time: 100 ms, 0.2 amu step
Fragmentor: 180 V
Drying gas: 10 L/min, 350 °C
Nebulizer pressure: 50 psig
Capillary voltage: 3500
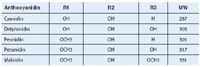
EICs for anthocynadins: Cyanidin m/z 286
Delphinidin m/z 302
Peonidin m/z 300
Petunidin m/z 316
Malvidin m/z 330
Four Agilent ZORBAX RRHD 2.1 × 100 mm, 1.8 µm columns were used:
1) Eclipse Plus C18 (p/n 959758-902)
2) Eclipse Plus Phenyl-Hexyl (p/n 959758-912)
3) StableBond SB-Aq (p/n 858700-914)
4) StableBond SB-Phenyl (p/n 858700-912)
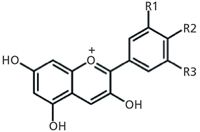
Figure 1: Chemical structures of five common anthocyanidins found in blueberry extract.
Results and Discussion
Ten grams of blueberries are extracted into acidified methanol and analysed by LC–MS. A rapid screening of these four columns is possible within this 1200 bar system and column pressure limit. With this methanol/formic acid gradient at 0.65 mL/min, the maximum pressure generated is 1020 bar, and each analysis is accomplished in only 15 min. Total ion chromatograms (TICs) are generated from a scan from 200–1000 with the blueberry extract on each column. Extracted ion chromatograms (EICs) reveal the glycosides and acylglocysides of five common anthocyanidins.

Figure 2: Extracted ion chromatograms from LCâMS scan data of blueberry anthocyanins.
The EICs in Figure 1 clearly show the distinct glycosides and acylglycosides of the five different anthocyandins, each marked with a unique colour. By looking at the EICs it is easy to see many of the smaller peaks in the chromatogram, several of which coelute with larger peaks which would not be recognizable with UV or TIC chromatograms. From these EICs, the total number of resolved anthocyanin peaks is determined and summarized in Table 1. This data shows that the Eclipse Plus Phenyl-Hexyl column resolves a few more anthocyanin peaks with this methanol/formic acid gradient than the other three phases. To see more details and exhibits, reference the full application note on www.agilent.com (publication 5990-8470EN).

Table 1: Total number of blueberry anthocyanin peaks resolved by four Agilent ZORBAX RRHD columns with a methanol/formic acid gradient.
References
1. R.L. Prior, S.A. Lazarus, G. Cao et al., "Identification of procyanidins and anthocyanins in blueberries and cranberries (vaccinium spp.) using high performance liquid chromatography/mass spectrometry" J Agric. Food. Chem. 49(3), 1270–6 (2001).
Agilent Technologies Inc.
5301 Stevens Creek Blvd., Santa Clara, California, USA
tel: (800) 227-9770 (Directory) fax: (866) 497-1134
Email: agilent_inquiries@agilent.com
Website: www.agilent.com/chem/bioHPLCproteins

New Study Reviews Chromatography Methods for Flavonoid Analysis
April 21st 2025Flavonoids are widely used metabolites that carry out various functions in different industries, such as food and cosmetics. Detecting, separating, and quantifying them in fruit species can be a complicated process.
Analytical Challenges in Measuring Migration from Food Contact Materials
November 2nd 2015Food contact materials contain low molecular weight additives and processing aids which can migrate into foods leading to trace levels of contamination. Food safety is ensured through regulations, comprising compositional controls and migration limits, which present a significant analytical challenge to the food industry to ensure compliance and demonstrate due diligence. Of the various analytical approaches, LC-MS/MS has proved to be an essential tool in monitoring migration of target compounds into foods, and more sophisticated approaches such as LC-high resolution MS (Orbitrap) are being increasingly used for untargeted analysis to monitor non-intentionally added substances. This podcast will provide an overview to this area, illustrated with various applications showing current approaches being employed.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)
















