Authentication of Traditional Chinese Prescriptions Using Comprehensive 2D-LC
The Application Notebook
This application note shows the comprehensive 2D-LC analysis of the traditional Chinese prescription Si-Wu-Tang and the individual herbs contained in Si-Wu-Tang. The possibility for authentication of traditional Chinese prescriptions is investigated.
The authentication of traditional Chinese prescriptions is a challenging task because of the highly complex nature and the natural variability of the herbs used in Chinese herbal medicine (CHM). Generally, chromatographic fingerprinting is regarded as an effective method for authentication (1–4). Si-Wu-Tang is composed of the four herbs Radix Angelicae Sinensis, Rhizoma Chuanxiong, Radix Paeoniae Alba, and Radix Rehmanniae Preparata. For each herb, characteristic components are detected in Si-Wu-Tang as a means of authentication. Additionally, changes following the omission and replacement of one herb from Si-Wu-Tang are examined.
Experimental Conditions
Comprehensive 2D-LC analysis was achieved with the Agilent 1290 Infinity 2D-LC Solution. In the first dimension an Agilent ZORBAX RRHT SB-Aq column (2.1 × 100 mm, 1.8-µm) was used with a gradient of water and methanol, each with 0.1% formic acid, at a flow rate of 0.05 mL/min. The second dimension separation used an Agilent Poroshell 120 Bonus-RP column (3.0 × 50 mm, 2.7-µm) with shifted gradients of water and acetonitrile, each with 0.1% formic acid, at a flow rate of 2.5 mL/min. Modulation was realized using the Agilent 2-position/4-port-duo valve, equipped with two 40 µL loops. A modulation time of 30 s was employed. Detection was performed at 254 nm as well as using QTOF mass spectrometry in positive and negative ionization mode. Samples were prepared as decoctions, in the same manner as they are prepared for pharmaceutical use.
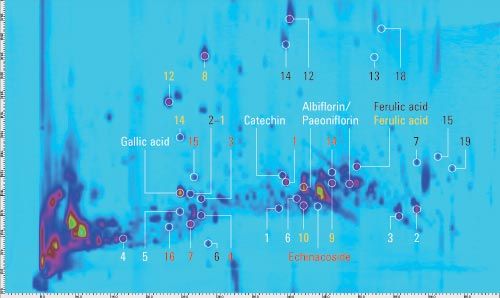
Figure 1a: Comprehensive 2D-LC analysis of a decoction from Si‑Wu‑Tang with MS detection in positive (a) and negative (b) ionization mode. Peaks matched from the templates of the individual herbs are marked: Radix Angelicae Sinensis (yellow), Rhizoma Chuanxiong (black), Radix Paeoniae Alba (white), Radix Rehmanniae Preparata (red).
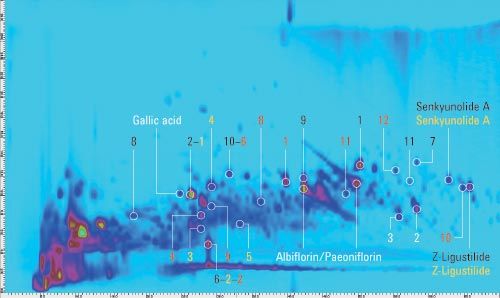
Figure 1b: Comprehensive 2D-LC analysis of a decoction from Si‑Wu‑Tang with MS detection in positive (a) and negative (b) ionization mode. Peaks matched from the templates of the individual herbs are marked: Radix Angelicae Sinensis (yellow), Rhizoma Chuanxiong (black), Radix Paeoniae Alba (white), Radix Rehmanniae Preparata (red).
Results
To enable authentication of Si-Wu-Tang, a separate comprehensive 2D-LC analysis of each herb contained in Si-Wu-Tang was performed. Detection of accurate masses by QTOF mass spectrometry in connection with literature data enabled the tentative identification of characteristic components of each herb. The peaks that were tentatively identified and further high abundant peaks were selected to construct a template for each herb. Each template was then matched to the peaks detected in Si-Wu-Tang in terms of first and second dimension retention times as well as agreement of the base peak in the respective mass spectra. Figure 1 shows the analysis of Si-Wu-Tang with the peaks matched from the templates of the individual herbs. Several peaks detected in Si-Wu-Tang can be attributed to more than one individual herb, for example, senkyunolide A from Radix Angelicae Sinensis and Rhizoma Chuanxiong.
Generally, 75% or more of the template peaks could be matched to peaks detected in Si-Wu-Tang. This shows the possibility to detect characteristic components of an individual herb in a traditional Chinese prescription. Additionally, adulteration through omission and replacement of one herb from Si-Wu-Tang could be detected by the matching of a considerably reduced number of template peaks.
Conclusions
Comprehensive 2D-LC is ideally suited for the analysis of complex samples such as the traditional Chinese prescription Si-Wu-Tang. The analysis of Si-Wu-Tang and its individual herbs provides a means of authentication. Further, it is illustrated that one or a few marker compounds for each herb are not sufficient for authentication when those compounds are not uniquely contained in one herb.
References
- P.S. Xie, and A.Y. Leung, Journal of Chromatography A 1216, 1933–1940 (2009).
- X.M. Liang et al., Journal of Chromatography A 1216, 2033–2044 (2009).
- D.Z. Yang et al., Journal of Chromatographic Science51, 716–725 (2013).
- Y.Z. Liang et al., Journal of Chromatography B812, 53–70 (2004).

Agilent Technologies Inc.
5301 Stevens Creek Blvd., Santa Clara, California 95051, USA
Website: www.agilent.com

Determining the Link Between Prenatal Cannabis Use and Symptoms of Depression Using LC–MS/MS
April 16th 2025Researchers investigating the relationship between cannabis use during pregnancy and depressive symptoms—and whether continued use beyond the first trimester or higher levels of use were linked to increased symptoms—used liquid chromatography–tandem mass spectrometry (LC–MS/MS) to confirm the presence of 11-nor-9-carboxy-delta-9-tetrahydrocannabinol (THC-COOH) in urine samples.
A Guide to (U)HPLC Column Selection for Protein Analysis
April 16th 2025Analytical scientists are faced with the task of finding the right column from an almost unmanageable range of products. This paper focuses on columns that enable protein analysis under native conditions through size exclusion, hydrophobic interaction, and ion exchange chromatography. It will highlight the different column characteristics—pore size, particle size, base matrices, column dimensions, ligands—and which questions will help decide which columns to use.
Common Challenges in Nitrosamine Analysis: An LCGC International Peer Exchange
April 15th 2025A recent roundtable discussion featuring Aloka Srinivasan of Raaha, Mayank Bhanti of the United States Pharmacopeia (USP), and Amber Burch of Purisys discussed the challenges surrounding nitrosamine analysis in pharmaceuticals.


















