Ultrafast UHPLC–MS–MS Method Development in Therapeutic Drug Monitoring
The Application Notebook
Shimadzu Europa GmbH
According to the World Health Organization (WHO), heart disease is the number one cause of death worldwide. As a result, medication for heart treatment is counted among the most frequently prescribed therapeutic classes. While most prescription drugs can cause some adverse reaction in a patient, side effects of cardiovascular agents can be particularly hard to manage. There may only be a subtle distinction between a therapeutic dose and a life-threatening one. Therefore, efficient drug monitoring is an important tool in enhancement of drug efficacy and reduction of the risk of toxic effects resulting in a balanced treatment.
With the advance of highly sensitive and fast liquid chromatography tandem mass spectrometry (LC–MS–MS) instruments, triple quadrupole technology has found its way into clinical drug monitoring. It is the preferred technique for an increasing number of applications in the clinical sector, demanding fast and efficient development of new LC–MS–MS methods. Fast ultrahigh-pressure liquid chromatography (UHPLC) screening using Shimadzu's specialized scouting software in combination with automated MS optimization for multiple reaction monitoring (MRM) parameters are the perfect platform for rapid generation of dedicated analytical procedures.
Experimental
For UHPLC method scouting, a Shimadzu Nexera X2 Method Scouting System was used, consisting of two quaternary solvent pumps (LC-30AD), an autosampler (Sil-30AC), and a column oven (CTO-20AC) including a six-column switching valve (FCV-34AH). The system was also equipped with a Shimadzu LCMS-8040 triple quadrupole mass spectrometer via an electrospray ionization (ESI) source.
Figure 1: Structures of cardiovascular drugs.
The method scouting system enables screening of a maximum of six HPLC columns with up to 16 different eluents. The different mobile and stationary phases used for method scouting for the separation of eight cardiovascular drugs are displayed in Table 1.
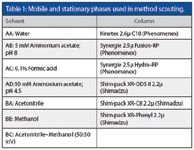
Table 1: Mobile and stationary phases used in method scouting.
For automated generation of an optimized MRM method the first step is selection of the precursor ion, followed by mass-to-charge ratio (m/z) adjustment of the precursor. The collision energy is optimized for the most abundant fragments and finally the fragment m/z is adjusted. These optimization steps were performed via flow injection analysis, each taking 30 s (Figure 2).

Figure 2: Automated multiple reaction monitoring (MRM) optimization on the LCMS 8040.
Method scouting was performed in a 30 h sequence using 5 min and 2 min gradient runs with varying gradient slope and all possible combinations of aqueous and organic mobile phases on the six columns specified in Table 1.
Results
A total of 162 different chromatographic conditions were evaluated for the best separation and peak intensities (Figure 3).
Final method:
Column: Synergie 2.5μ Hydro-RP, 100 × 2.00 mm (Phenomenex)
Flow rate: 0.4 mL/min
Temperature: 50 °C
Solvent A: 5 mM Ammonium acetate, pH 8
Solvent B: Methanol
Gradient: 30–85%B in 5 min, 5.01 min to 95%B, 3 min hold, 2 min post time

Figure 3: (a) Shim-pack C18, 5â95% BA in AC in 5 min; (b) Synergie Hydro-RP, 25â85% BB in AB in 2 min; (c) Synergie Hydro-RP, 30-85% BB in AB in 5 min.
Conclusion
The Nexera X2 method scouting system in combination with Shimadzu's ultrafast LCMS 8040 triple quad mass analyser is a unique tool for quick and efficient development of LC–MS–MS applications. Chromatographic separation of eight cardiovascular drugs as well as their identification and quantification was established successfully within two working days.

Shimadzu Europa GmbH
Albert-Hahn-Str. 6–10, D-47269 Duisburg, Germany
Tel: +49 203 76 87 0 fax: +49 203 76 66 25
E-mail: shimadzu@shimadzu.com
Website: www.shimadzu.eu

Polysorbate Quantification and Degradation Analysis via LC and Charged Aerosol Detection
April 9th 2025Scientists from ThermoFisher Scientific published a review article in the Journal of Chromatography A that provided an overview of HPLC analysis using charged aerosol detection can help with polysorbate quantification.
Analyzing Vitamin K1 Levels in Vegetables Eaten by Warfarin Patients Using HPLC UV–vis
April 9th 2025Research conducted by the Universitas Padjadjaran (Sumedang, Indonesia) focused on the measurement of vitamin K1 in various vegetables (specifically lettuce, cabbage, napa cabbage, and spinach) that were ingested by patients using warfarin. High performance liquid chromatography (HPLC) equipped with an ultraviolet detector set at 245 nm was used as the analytical technique.




















