Analysis of Barbiturates in Urine with Agilent 6430 LC–MS–MS and Poroshell 120 EC-C18
The Application Notebook
Toxicology Laboratory at the Veterans Administration, Portland, Oregon, USA, Agilent Technologies
A fast, cost-effective, and highly sensitive method was developed for the determination of five barbiturates with four internal standards (ISTDs) in urine using an Agilent 6430 Triple Quadrupole LC–MS system and an Agilent Poroshell 120 EC-C18 column. The sample of urine was extracted using an Agilent SPEC-C18AR cartridge. Results indicate that the method effectively extracts the selected barbiturates from urine, resolving the target compounds and the ISTDs in 8.5 min. Though amobarbital and pentobarbital differ only in the position of a methyl group and are, therefore, difficult to separate, their resolution is sufficient for routine analysis.
Materials and Methods
All compounds were purchased from Cerilliant Corporation, Round Rock, Texas, United States.
Sample Preparation
1. Begin by centrifuging the urine sample at 2800 rpm for 5 min.
2. Pipette 1 mL of centrifuged sample into a 13 mm × 100 mm borosilicate glass tube.
3. Add exactly 35 µL of the working deuterated internal standard (butalbital-D5, pentobarbital-D5, secobarbital-D5, and phenobarbital-D5).
4. Pipette 500 µL 0.1 M phosphate buffer into the sample. The phosphate buffer is prepared by adding 13.61 g KH2PO4 into 800 mL water, adjusting to pH 6.0 with KOH, then making the volume up to 1 L.
5. Use a vacuum chamber with the Agilent SPEC-C18AR cartridge for extraction. Condition the cartridge with 0.2 mL of MeOH and load the sample solution.
6. Wash the column with 0.5 mL water and dry for 1 min.
7. Elute the cartridge with 1 mL 90:10 hexane:ethyl acetate mixture.
8. Collect the eluent and dry the sample under nitrogen gas at 35 °C.
9. Reconstitute with 0.5 mL 90:10 water:acetonitrile mixture.
HPLC Conditions
The method was performed on the Agilent 1260 Infinity LC with a 6430 Triple Quadrupole LC–MS.
Column: Agilent Poroshell 120 EC-C18, 2.1 mm × 100 mm, 2.7 µm (p/n 695775-902)
Sample preparation: Agilent SPEC-C18CR, 3 mL, 15 mg (p/n A5321920); Eluent A, 5 mM ammonium acetate; Eluent B, LC–MS grade acetonitrile
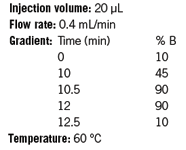
MS Conditions
ESI drying gas: 350 °C, 10 L/min
Nebulizer: 40 psi
Negative ionization mode
Capillary: 4000 V, DEMV 400
Note: If using an instrument that is not configured for low delay volume, use about 1 minute initial hold.

Figure 1: MRM chromatograms of barbiturates and internal standards using an Agilent Poroshell 120 EC-C18 column.
Results and Discussion
The superficially porous particles of Poroshell 120 have nearly identical efficiency as sub-2 µm totally porous materials and therefore can be used to provide similarly fast and high resolution analyses at a lower pressure. A separation of the nine barbiturates in 8.5 min was achieved on the column with a gradient method (Figure 1). Reasonable resolution was achieved between the standard components, except for pentobarbital and amobarbital. These are the isomers with the same product ions, which could not be identified by MS. However, they still have some separation on Poroshell 120 EC-C18 and the resolution for amobarbital and pentobarbital is sufficient for routine analysis.
Linearity and Recovery
The stock standards solution, containing phenobarbital, butalbital, pentobarbital, amobarbital, and secobarbital, was diluted to a series of linear solutions of 3000 ng/mL, 1500 ng/mL, and 150 ng/mL. In each solution, the ISTDs of phenobarbital-d5, butalbital-d5, pentobarbital-d5, and secobarbital-d5 were made up to a concentration of 1000 ng/mL. The method showed excellent linearity, being very close to 1.0 (from 0.9995 to 0.99998). For more information, including calibration curves, see the full-length application note, Agilent publication 5991-2596EN.
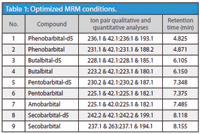
Table 1: Optimized MRM conditions.
The standards, at a concentration of 150 ng/mL, were spiked into the urine sample blank and processed with the solid-phase extraction (SPE) procedure. The recoveries were calculated and are shown in Table 2.
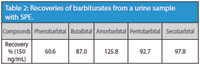
Table 2: Recoveries of barbiturates from a urine sample with SPE.
Conclusions
A method was developed for the extraction and separation of barbiturates using an Agilent SPEC-C18AR for sample extraction and an Agilent Poroshell 120 EC-C18 column for separation. The sample preparation method effectively extracted the selected barbiturates from urine, with sufficient recoveries and precision. The column provided good selectivity and good resolution for these compounds. The method developed on the Agilent 6430 Triple Quadrupole LC–MS system was suitable for quantitative analysis of these compounds in urine, especially at low concentration levels.

Agilent Technologies
2850 Centerville Road Wilmington, Delaware 19808, USA
Website: www.agilent.com

Polysorbate Quantification and Degradation Analysis via LC and Charged Aerosol Detection
April 9th 2025Scientists from ThermoFisher Scientific published a review article in the Journal of Chromatography A that provided an overview of HPLC analysis using charged aerosol detection can help with polysorbate quantification.
Analyzing Vitamin K1 Levels in Vegetables Eaten by Warfarin Patients Using HPLC UV–vis
April 9th 2025Research conducted by the Universitas Padjadjaran (Sumedang, Indonesia) focused on the measurement of vitamin K1 in various vegetables (specifically lettuce, cabbage, napa cabbage, and spinach) that were ingested by patients using warfarin. High performance liquid chromatography (HPLC) equipped with an ultraviolet detector set at 245 nm was used as the analytical technique.
Removing Double-Stranded RNA Impurities Using Chromatography
April 8th 2025Researchers from Agency for Science, Technology and Research in Singapore recently published a review article exploring how chromatography can be used to remove double-stranded RNA impurities during mRNA therapeutics production.



















