Simultaneous Determination of Positive and Negative Counterions Using a Hydrophilic Interaction Chromatography Method
LCGC North America
The authors work to develop a universal high performance liquid chromatography method that is capable of simultaneously retaining and separating both cations and anions within a single chromatographic analysis for the purpose of quantification in pharmaceutical products.
The objective of this work was to develop a universal high performance liquid chromatography method that is capable of simultaneously retaining and separating both cations and anions within a single chromatographic analysis for the purpose of quantification in pharmaceutical products. A zwitterionic stationary phase operated in the hydrophilic interaction chromatography (HILIC) mode in conjunction with evaporative light scattering detection was investigated for the separation and quantitation of 33 commonly used pharmaceutical counter ions, 12 cations, and 21 anions. Using a single gradient chromatographic analysis, both anions and cations were easily separated from each other in addition the parent pharmaceutical molecules also were separated. The zwitterionic stationary phase utilized in this study offers unique separation capabilities based upon its mixed-mode separation mechanism (that is, electrostatic ion chromatography with the positively and negatively charged functional groups on the stationary phase and HILIC). As a result, a generic screening method was devised that allows for counterion determinations regardless of the pharmaceutical salt that is investigated. The unique retention characteristics of this column were evaluated by varying key mobile phase parameters, such as pH, buffer strength, and organic modifier. After examining the changes in retention, response, and resolution, this universal method was then further evaluated for reproducibility for multiple counterion determinations. For counterion determinations, a typical precision of <2.0% was observed for all counterions and most determinations were within 2.5% of the theoretical salt concentration. Thus, a very rugged screening method was developed capable of separating both anions and cations within a single chromatographic analysis. Counterion determinations were demonstrated for 10 pharmaceutically relevant salts.
The separation and quantitation of counterions in the pharmaceutical industry is an important determination. During drug development, the selection of the correct salt form early in the development process can prevent repeating toxicology, biological, and stability studies. As a result, development timeline delays can potentially be prevented. The initiation of the salt selection process generally takes place for all ionizable compounds that successfully have passed initial toxicology screening. The most common pharmaceutical salt forms are sodium salts of acids and hydrochloride salts of amines. Ideally, these salts would be nonhygroscopic, exhibit solid–state stability, and possess high aqueous solubility. However, the most common salt forms do not always possess the best physicochemical properties and attributes for development success. In these cases, a multidisciplinary salt-selection process is necessary to find alternative acceptable salt forms. Automated salt selection systems can be used to screen numerous counterions in various solvent systems, which can result in atypical salt forms. The salt forms that are crystalline from this screen will be scaled up for further evaluation. At this point, the analyst typically evaluates the salt forms using high performance liquid chromatography (HPLC) for counterion identity and stoichiometry confirmation. The final salt that proceeds into clinical trials typically has desirable properties in relation to stability, bioavailability, and is most amenable to conventional formulation development. The method of counterion determination needs to be precise, accurate, and rugged so that it easily can be transferred to other analytical laboratories where the active pharmaceutical ingredient is routinely monitored to ensure the safety, identity, strength, purity, and quality of the material. This material ultimately will be made into a drug product and consumed by the patient.
Several options exist for counterion determinations. The most commonly employed determination utilizes ion-exchange chromatography (IC), which was introduced in 1975 (1). In IC, conductivity detection is typically used and a suppressor is required to reduce the background signal. Over the last 30 years, IC with conductimetric detection has proven to be a very sensitive detector for both cations and anions. However, to perform a cation separation, for example, a cation exchange column with a cation suppressor is required to get adequate sensitivity. The same is true for anions, but utilizes an anion exchange column and suppressor. An alternative approach would employ strong anion or strong cation exchange columns in conjunction with UV detection for the determination of organic acids, or evaporative light scattering detection (ELSD) for detection of inorganic salts. Capillary electrophoresis (CE) also has been shown to be useful for counterion analysis and a method for simultaneous determination of anionic metabolites based upon CE–mass spectrometry (MS) has been shown to be specific and selective (2).
In general, all of the previous methodologies involve more than one column, more than one mobile phase, and in many cases more than one mode of detection to determine both cations and anions. An ideal, and sometimes necessary situation would allow for the separation of anions and cations within a single chromatographic run. For example, a zwitterionic compound proceeding through salt selection can form a basic or acidic salt form. When only milligram quantities of material are available, a single method of separating both cations and anions would allow for identity, salt confirmation, and stoichiometry within a single chromatographic run.
The concept of electrostatic ion chromatography (EIC), or zwitterionic ion chromatography (ZIC) as it was later named, with a zwitterionic stationary phase for the separation of ions, was first proposed by Hu and colleagues in 1993 (3). This separation principal is based upon a zwitterionic stationary phase that maintains a fixed positive and negative charge in close proximity to each other. The separation relies on the ability of the analyte ions to access both the fixed positive charge, in the case of anions, and the fixed negative charge, in the case of a cation. As a result of the proximity of the charges, the analyte ions will be repulsed and attracted at the same time. Thus, a unique and sometimes complicated selectivity is obtained. Many mechanistic studies have been performed that attempt to outline the charge interactions on a molecular level. Hu and Haddad reported the formation of an electrical double layer (4,5) to explain retention mechanisms. Okada and Patil modeled zwitterionic retention based upon Poisson–Boltzmann theory (6). The formation of a Donnan membrane combined the previous theories of Hu (electric double layer) and Patil (charged surfaces) to explain both elution order and the effect that mobile composition has on retention (7,8). However, there have been few applications reported that take full advantage of the separating power of this unique stationary phase.
Many of the early applications have utilized pure water as the mobile phase, and as a result have had difficulty separating both anions and cations. A sulfobetaine stationary phase was reportedly not successful in the simultaneous separation of inorganic cations (9). In this study, it was noted that the simultaneous repulsion and attraction forces prevented the anions and their countercations from achieving an ion exchange interaction. Thus, the anion is coeluted with its cation. In a later investigation of a slightly modified zwitterionic system (that is, different carbon chain length between charges), simultaneous separation of cations and anions was successfully performed (10). Again, an aqueous eluent with perchlorate–perchloric acid modifier was chosen because it provided the best separation.
Recently, a carboxybetaine zwitterionic column was evaluated for the analysis of nutrients in seawater (11). In addition, the effect of electrolyte concentration (KCl) and pH were demonstrated to have an effect on anion retention. However, the mechanism was viewed as more complicated than simple ion exchange. In a separate evaluation of a carboxybetaine zwitterionic stationary phase (12), several retention trends were documented. First, both the positively and negatively charged groups impact the separation of anions, whereas cations mainly interact with the negatively charged group. The interaction of anions with the positively charged group is influenced by the cation in the mobile phase, but mainly follows anion-exchange principles. A sulfobetaine-type zwitterionic stationary phase, similar to that used in this investigation, using water as a mobile phase, was evaluated for the separation of multiple anions (13). This study indicated that anions with large hydration energies could not be separated because they have very little retention. The experiments conducted here will demonstrate that organic modifier can play a key role in the retention of these molecules based upon the facilitation of hydrophilic interaction chromatography (HILIC). Jonsson and Appelblad demonstrated the separation of polar and hydrophilic compounds with a sulfobetaine-type zwitterionic stationary. This work focused on the selectivity from a HILIC perspective, where the effect of acetonitrile and methanol was evaluated for the retention of RNA–DNA bases in an ammonium formate buffer system (14).
The approach presented here also uses a zwitterionic column operated in the hydrophilic interaction chromatography (HILIC) mode with evaporative light scattering detection (ELSD). The combination of separation mechanisms (that is, HILIC and EIC) can, theoretically, complicate the understanding of the separation mechanism; however, the utility of the zwitterionic column is greatly enhanced with the addition of organic to the mobile phase to take advantage of the HILIC effect. Alpert first coined the term hydrophilic interaction chromatography for the separation of proteins, peptides, and polar molecules (15), although this mechanism had been previously established for the separation of carbohydrates (16,17). The HILIC mode employs polar stationary phases with mixed aqueous–organic mobile phases creating a stagnant enriched water layer around the polar stationary phase. This enriched layer allows analytes to partition between the two phases based upon their polarity. In contrast to reversed-phase chromatography, where a nonpolar stationary phase is employed and analyte elution is facilitated by the organic strength of the mobile phase, analyte elution is facilitated by the aqueous (more polar) component of the mobile phase in HILIC mode. The separation mechanism and retention order in the HILIC mode is therefore opposite to that of the reversed-phase mode. Although the HILIC mode is more similar to the normal phase and polar organic modes, it is different in that the HILIC mobile phases contain a relatively high amount of water (typically 5–40%) as the strong eluent, which can provide a significant solubility advantage for very hydrophilic samples. The HILIC mode can be generated by a variety of polar stationary phases. Examples are piperazine which has been determined utilizing the HILIC mode on a cyano column (18) and polar pharmaceutical analytes which have been separated using both amino and silica columns (19). The HILIC mode also has been employed for chiral separations using cyclodextrin and macrocyclic antibiotic based packings (20,21).
In HPLC, ELSD has an extensive application base, but it is especially important when UV detection is not feasible. The concept and operation of commercially available evaporative light-scattering detectors as sensitive and universal has been discussed thoroughly in the literature (22). ELSD has been shown to successfully detect many substances, such as phospholipids (23–26), triglycerides, fats and fatty acid esters (27,28), carbohydrates (29–31), synthetic polymers (32), steroids (33), and amino acids (34,35). The HPLC–ELSD system also has been extremely useful for the determination of pharmaceutical impurities, raw materials, cleaning verification and small organic compounds (36–39). A more recent niche for ELSD in the pharmaceutical industry is for the detection and quantitation of counterions from pharmaceutical salt forms. Our laboratory first introduced the applicability of HPLC–ELSD for the detection and quantitation of inorganic ions, such as chloride and sodium (40–42). A comparison of the HPLC–ELSD technique with ion chromatography, capillary electrophoresis, and titration for the determination of Cl- in pharmaceutical drug substances has been compared statistically and it was determined that the four techniques were equivalent (41). However, ELSD is a cost effective method that can be used with many other HPLC applications in addition to the analysis of counterions (for example, assay and impurity determinations for compounds lacking a strong chromophore) which gives it a unique advantage over other techniques.
The goal of this article is to show the application of a relatively new column technology operated in the HILIC mode, while fully taking advantage of the EIC interaction, for the simultaneous separation and quantitation of cations and anions within a single chromatographic run with ELSD as a universal detection system.
Experimental
Chemicals: Acetonitrile was purchased from Burdick and Jackson (Muskegon, Michigan). A sodium and chloride standard solution was acquired from Fluka Chemika (Buchs, Switzerland). The pH buffers were from Red Bird Service (Osgood, Indiana). Deionized water and nitrogen were from an in-house system. All other chemicals were obtained from Sigma-Aldrich Chemical Company (St. Louis, Missouri).
Equipment: The HPLC system consisted of a Hewlett Packard 1050 pump and auto sampler (Wilmington, Delaware) integrated with an Alltech 800 evaporative light scattering detector from Alltech Associates (Deerfield, Illinois). The detector was operated at 55 °C, 3.5 bar nitrogen and a gain setting of 1 throughout the experiments. A ZIC-HILIC column (250 × 4.6 mm, 5 μm) from SeQuant was used for the separation (Umea, Sweden). The mobile phase flow rate was set at 1.0 mL/min and injection volumes of 10 or 20 μL were used. Mobile phase A make-up was 85% acetonitrile–15% buffer and mobile phase B was 10% acetonitrile–90% buffer. The buffer comprised ammonium acetate and pH adjusted with acetic acid (buffer concentration and pH were varied and noted in the text). An Orion model 720A pH meter from Orion Research, Inc. was used to measure the pH of the mobile phase buffers (Beverly, Massachusetts). The gradient system employed with each injection was as follows: 0–2 min at 100% A, 2–22 min a linear gradient to 100% B, 22–25 min at 100% B, 25–26 min a linear gradient back to 100% A, and equilibrate 26–35 min at 100% A. Note that this gradient is opposite of conventional reversed-phase HPLC due to the fact that HILIC is employed.
Standard and sample preparation: Three individual standards were weighed accurately and diluted with mobile phase A or accurately pipetted from a standard stock solution and diluted with mobile phase A. The standard curve for three calibration standards was calculated by least-squares regression analysis of peak area versus concentration. The samples were weighed individually and the weights were based upon the theoretical content of the counterion to be within the standard range. The concentration of the counterion in the samples was determined by comparing the peak area to the standard curve.

Figure 1
Results and Discussion
Effect of Organic Composition: The first important aspect of this work was to establish whether the zwitterionic stationary could exhibit a HILIC effect for the separation of inorganic cations and anions. A concern was that the electrostatic effect would dominate the separation and the organic modifier would have very little effect on the selectivity. As can be seen in the bottom chromatographic trace in Figure 1, Na+ and Cl- are not separated with 20% acetonitrile–80% buffer. As the organic content of the mobile phase is increased from 20% to 80% acetonitrile, the retention times of both ions are increased substantially from 3.5 min to 10.5 min for the chloride ion and to approximately 20 min for the sodium ion. In addition, the resolution between the ions increases with increased organic composition. In a typical reversed-phase interaction (not that group I cations are retained typically on a reversed-phase column), these ions would be eluted in the solvent front for all mobile phase compositions. In the same fashion, in a completely aqueous system with a zwitterionic stationary phase, these ions would have been coeluted, which had been reported previously as an ion-pairing effect (43). Therefore, this is a strong indicator that organic composition of the mobile phase is an extremely powerful tool in controlling selectivity and retention of anions and cations in the HILIC mode. For all of the investigation in this work, acetonitrile was used because it already has been demonstrated that acetonitrile will promote the HILIC effect more so than methanol. In addition, Figure 1 also illustrates that even for a simple separation of Na+ and Cl- ; the run time can become excessively long. Thus, a gradient (that is, opposite of a typical reversed-phase separation) will be used for all future separations with the understanding that for any compound–counterion separation, the run time could be optimized for an isocratic separation. The data generated will be a gradient ramped from 15% aqueous buffer to 90% aqueous buffer.
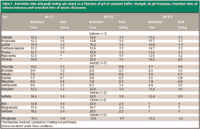
Table I
Effect of pH: pH effects were evaluated across a range of approximately 3.1–6.6. Across this range the sulfobetaine-type zwitterionic stationary phase retains its permanent positive and negative charges. Because there is no change in ionization state of the analyte ions (for the inorganic ions) or stationary phase across the pH range, it was presumed that pH differences would have a minimal effect on ion retention. However, a definite trend was observed. These experiments were conducted with constant buffer strength of 50 mM ammonium acetate. The mobile phase starting point in this case was 85% acetonitrile–15% buffer (pH was adjusted with acetic acid) and a flow rate of 1 mL/min was used (see equipment section for gradient).
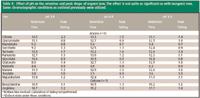
Table II
A measurable effect on retention is observed as the pH is increased from 3.1 to 6.6 for both cations and anions. Interestingly, as the pH was increased, the retention times of all of the cations increased and the retention times of the anions decreased (see Table I). The change in retention was most drastic for the +2 cations where calcium, magnesium, and zinc were not eluted under these gradient conditions at pH 6.6. The effect on cation retention is presumably due to the H+ interacting with the negatively charged part of the zwitterions (SO3- ), which ultimately shields the cation from having a strong interaction at a lower pH. The anions are following standard ion exchange theory. As can be seen in Table I, there is a minimal effect of pH on peak shape except for the +2 ions. In this case, a lower pH is recommended to ensure that the ions will be eluted and better peak shape will be obtained. The separation of Na+ and K+ is fairly difficult under these conditions. At pH 3.1, these two cations essentially are coeluted and at pH 6.6 they are slightly separated with a retention time difference of approximately 30 s.
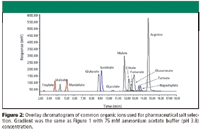
Figure 2
For organic ions, the trends (see Table II) are not as clearly defined due to the pKa of the acids. Dependent upon the ionization state of the ion, the retention mechanism could change from ion exchange to one that is affected by hydrogen bonding. However, a reasonable separation is obtained for multiple (14) organic ions that are commonly used to synthesize pharmaceutical salts (Figure 2). Figure 3 represents the separation of mainly the inorganic ions that were evaluated within this work (see Table I). Interestingly, ions of a particular charge state are eluted within distinct regions of the chromatogram under these separation conditions. For example, Figure 3 demonstrates that the elution order is, in general -1<+1<-2<-3<+2. The -2 and -3 anion elution order is predicted only from SO4-2 and PO4-3 and is not as reliable as the predictions that do not include polyatomic ions (for example, lysine elutes after sulfate and phosphate). However, this elution order is a powerful tool in understanding the interactions that dominate the separation. For example, all -1 ions are eluted before all +1 ions, which indicates that the fixed SO3- functionality on the stationary phase has a strong interaction with cations because it is more accessible.
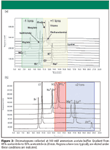
Figure 3
Effect of buffer concentration: Because ELSD was used in this investigation, a volatile buffer of some sort was required for the detection of the ions. For example, ammonium acetate buffer can be used in the mobile phase so that a particle of Na+ CH3COO- will be formed during desolvation in the detector drift tube and subsequently detected by light scattering. In the case of Cl- , under these same conditions, NH4+ Cl- is formed and subsequently detected. In this study, ammonium formate and ammonium acetate were evaluated. Ammonium formate offered no advantages over ammonium acetate. Therefore, an ammonium acetate–acetonitrile system was evaluated for all experiments. In addition to allowing for the detection of ions by ELSD, the buffer concentration has a pronounced effect on the chromatography, and in combination with organic concentration appears to be the most important variable in controlling selectivity. As can be seen in Table III, when the buffer concentration is increased from 10 mM to 200 mM ammonium acetate, both the peak shape and retention times of the ions are drastically affected. This experiment was run with a gradient from 85% acetonitrile–15% ammonium acetate to 10% acetonitrile–90% ammonium acetate at approximately pH 5 at a flow rate of 1 mL/min (gradient described in experimental section).
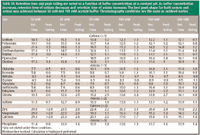
Table III
As expected, and reported previously, the buffer concentration has a significant impact on retention and peak shape of ions. The trend observed while increasing buffer concentration from 10 mM to 200 mM was that cations were not retained as long, and anion retention increased. Again, this can be explained by a two-part mechanism.
Because the main mechanism of interaction in the HILIC mode is based upon a partitioning of the ions into the aqueous phase that forms a stagnant layer on the stationary phase surface, the decrease in retention time might be best understood by a shift in equilibrium concentrations. As the NH4+ concentration increases preferentially in the aqueous layer, there is less opportunity for the analyte counterions to partition into the aqueous layer (44). Thus, the ions are swept through the column (mainly in the organic layer) with less interaction with the column and the aqueous phase. In addition, as the NH4+ interacts strongly with the SO3- fixed negative charges as the buffer concentration increases, access to these fixed charges is diminished. As a result, cations do not interact with SO3- and are not significantly retained; anion retention is affected in the opposite manner. The anions do not experience the typical repulsion forces of the SO3- functionality and can then access the tertiary amine for ion exchange. This ion exchange interaction causes the anions to be retained more strongly.
In addition to retention time effects, buffer concentration also impacts peak shape. With a very low buffer concentration (10 mM), the peak shapes exhibited severe fronting for the anions and in most cases tailing for the cations. As the buffer concentration was increased to 100 mM, the peaks symmetry improved, however, there were no improvements beyond that point. At 200 mM, the +1 cations actually began to exhibit significant peak fronting. From this experiment, a range of 50 mM–100 mM buffer concentration was a recommendation for these experiments. Phosphate buffer was considered so as to allow for UV detection of the organic acids, however, the solubility in high organic is limited and also diminishes further as the pH of the aqueous portion increases (when mixed with acetonitrile).
The retention of all ions investigated, in most cases beyond 4 min, is very convenient for counterion determinations. When operating in HILIC mode nonpolar compounds will be eluted with very little retention because they are portioned preferentially into the organic layer. Even though these compounds will be charged in many cases, the organic content will dominate the retention mechanism and the compound should be eluted before counterions.
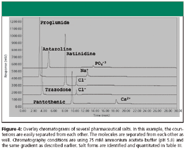
Figure 4
Counterion determination: This work was concluded by evaluating the zwitterionic column operated in the HILIC mode, in conjunction with ELSD, by determining the counterion concentration in 10 pharmaceutically relevant salts. trazodone HCl, ranitidine HCl, imipramine HCl, verapamil HCl, and chlorpromazine HCl were chosen as the representative hydrochloride salts. Proglumide Na, antazoline phosphate, pantothenic acid Ca, fenoterol HBr, and enapril maleate also were evaluated. A gradient was again employed to demonstrate the resolving power and the utility of a universal method for separation of a counterion from the parent molecule. A starting mobile phase of 85% acetonitrile–15% 75mM ammonium acetate (pH 4.8 with acetic acid) with a 2-min hold, and gradient to 90% aqueous buffer were chosen based upon previous data (see experimental section for gradient). The linearity of standards was first evaluated. Excellent linearity (typical R2 of >0.999) of a three point standard was observed for all ions that were quantitated. The same calibration curve was utilized for all of the HCl salts. Standards were typically prepared in the range of 0.2–0.7 mg/mL of the counterion, while the samples were prepared in a concentration to fall within the standard range. As can be seen from Figure 4, the compounds are separated from each other under these conditions as well as all of the counterions. Again, this demonstrates the power of the gradient ZIC-HILIC effect as a universal screening method. The counterion-determination data are summarized in Table IV for multiple salts. The RSD for all measurements was less than 2.0% for three replicates and the maximum absolute difference between the theoretical salt concentration and the experimentally determined value was 2.8% for antazoline phosphate. In the case of the phosphate salt, which exhibited the largest error from theory, the difference could be attributed to the low solubility of phosphate in high organic concentrations, although this sample was not investigated further. However, most errors were within 0.3% absolute, which is consistent to previously reported quantitative data (41).
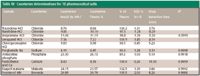
Table IV
Conclusion
This work demonstrates the separating power of the zwitterionic stationary phase for the simultaneous retention and separation of cations and anions, especially when organic eluent is used in the mobile phase. The column was successfully demonstrated to operate in the HILIC mode as a retention mechanism, where the retention times of Cl- and Na+ were increased from 3.5 min to 10.5 min and 20 min, respectively. The pH of the mobile phase had an effect where the retention of the cations was decreased and the retention of anions was increased. With the exception of the +2 cations, which required lower pHs to be eluted, the use of pH is considered more of a means to improve peak shape as opposed to control selectivity. Buffer concentration, as suspected with EIC interactions, is the most important parameter (in combination with organic content) for controlling selectivity, resolution, and for optimizing run times. These experiments clearly indicate that a large number of cations (12) and anions (21) can be separated and ultimately detected by ELSD. Finally, the gradient was applied to 10 pharmaceutical salts for the determination of the counterion. In this experiment, excellent linearity was observed with an R2 > 0.999 in most cases. There was excellent agreement with theory for all of the counterion determinations with most values within 2.5% of the theoretical salt concentration. In summation, a universal set of HPLC conditions with one column, one mobile phase, and one detection system, was developed that would suffice for the determination of a large population of pharmaceutically relevant salts.
References
(1) H. Small, T.S. Stevens, and W. Bauman, Anal. Chem. 47(11), 1801–1809, (1975).
(2) T. Soga, Y. Ueno, H. Naraoka, Y. Ohashi, M. Tomita, and T. Nishioka, Anal. Chem. 74(10), 2233–2239 (2002).
(3) W. Hu, T. Takeuchi, and H. Haraguchi, Anal. Chem. 65(17), 2204–2208 (1993).
(4) W. Hu, Langmuir 15(21), 7168–7171 (1999).
(5) W. Hu and P.R. Haddad, Trends in Anal. Chem. 17(2), 73–79 (1998).
(6) T. Okada and J. M. Patil, Langmuir 14(21), 6241–6248, (1998).
(7) H.A. Cook, W. Hu, J.S. Fritz, and P.R. Haddad, Anal. Chem. 73, 3022–3027 (2001).
(8) H.A. Cook, G. Dicinoski, and P.R. Haddad, J. Chromatogr., A 997(1-2), 13–20 (2003).
(9) W. Hu and H. Haraguchi, Anal. Chem. 66(5), 765–767 (1994).
(10) W. Jiang and K. Irgum, Anal. Chem. 71(2), 333–344 (1999).
(11) C.O. Riordain, P. Nesterenko, and B. Paul, J. Chromatogr. A 1070(1-2), 71–78 (2005).
(12) W. Hu, P.R. Haddad, K. Tanaka, and K. Hasebe, Anal. Bioanal. Chem. 375(2), 259–263 (2003).
(13) T. Umemura, S. Kamiya, A. Itoh, K. Chiba, and H. Haraguchi, Analytica Chimica Acta 349(1–3), 231–238 (1997).
(14) T. Jonsson and P. Appelblad, LCGC 17(Suppl.), 72-73 (2004).
(15) A.J. Alpert, J. Chromatogr. 499, 177–196 (1990).
(16) L.A. Th. Verhaar, and B.F.M. Kuster, J. Chromatogr., 234 (1), 57–64 (1982).
(17) P. Orth and H. Englehardt, Chromatographia, 15(2), 91–96 (1982).
(18) C. McClintic, D.M. Remick, J.A. Peterson, and D.S. Risley, J. Liq. Chromatogr. Rel. Technol. 26(18), 3093–3104 (2003).
(19) B.A. Olsen, J. Chromatogr. A 913 (1-2), 113–122 (2001).
(20) S.C. Churms, J. Chromatogr. A 720(1–2), 75–91 (1996).
(21) D.S. Risley and M.A. Strege, Anal. Chem. 72(8), 1736–1739 (2000).
(22) A. Stolyhwo, H. Colin, and G. Guiochon, J. Chromatogr. 265(1), 1–18 (1983).
(23) W.S. Letter, J. Liq. Chromatogr. 15(2), 253–266 (1992).
(24) J.S. Perona and V. Ruiz-Gutierrez, J. Sep. Sci. 27(9), 653–659 (2004).
(25) S.L. Abidi and T.L. Mounts, J. Chromatogr. A 773(1–2), 93–101 (1997).
(26) F. Mancini, E. Miniati, and L. Montanari, Italian J. Food Sci. 9(4), 323–336 (1997).
(27) A. Stolyhwo, M. Martin, and G. Guiochon, J. Liq. Chromatogr. 10(6), 1237–1253 (1987).
(28) T. Andersen, A. Holm, I. L. Skuland, R. Trones, and T. Greibrokk, J. Sep. Sci. 26(12–13), 1133–1140 (2003).
(29) R. Macrae, L.C. Trugo, and J. Dick, Chromatographia 15(7), 476–478 (1982).
(30) B.A. Kimball, W.M. Arjo, and J.J. Johnston, J. Liq. Chromatogr. Rel.Technol. 27(12), 1835–1848 (2004).
(31) Y. Wei and M.Y. Ding, J. Chromatogr. A, 904(1), 113–117 (2000).
(32) P.D. Green, H. Meng, and J.E. Seely, Polymer Preprints (American Chemical Society, Division of Polymer Chemistry) 38(1), 608–609 (1997).
(33) P.A. Asmus and J.B. Landis, J. Chromatogr. 316, 461–472 (1984).
(34) J.A. Peterson, L.J. Lorenz, D.S. Risley, and B.J. Sandmann, J. Liq. Chromatogr. Rel. Technol. 22(7), 1009–1025 (1999).
(35) H.J. C. Das Neves, and Z.B. Morais, J. High Resolut. Chromatogr. 20(2), 115–118 (1997).
(36) D.S. Risley and J.A. Peterson, J. Liq. Chromatogr. 18(15), 3035–3048 (1995).
(37) M. Rajevic and P. Betto, J. Liq. Chromatogr. Rel. Technol. 21(18), 2821–2830 (1998).
(38) D.S. Risley, K.F. Hostettler, and J.A. Peterson, LCGC 16(6), 562–568 (1998).
(39) C.E. Kibbey, Mol. Diversity 1(4), 247–258 (1996).
(40) J.A. Peterson and D.S. Risley, J. Liq. Chromatogr. 18(2), 331–338 (1995).
(41) D.S. Risley, J.A. Peterson, K.L. Griffiths, and S. McCarthy, LCGC 14(12), 1040–1047 (1996).
(42) M.D. Lantz, D.S. Risley, and J.A. Peterson, J. Liq. Chromatogr. Rel. Technol. 20(9), 1409–1422 (1997).
(43) W. Hu, H Tao, and H. Haraguchi, Anal. Chem. 66(15), 2514–2520 (1994).
(44) B.W. Pack and D.S. Risley, J. Chromatogr. A 1073(1–2), 269–275 (2005).

Common Challenges in Nitrosamine Analysis: An LCGC International Peer Exchange
April 15th 2025A recent roundtable discussion featuring Aloka Srinivasan of Raaha, Mayank Bhanti of the United States Pharmacopeia (USP), and Amber Burch of Purisys discussed the challenges surrounding nitrosamine analysis in pharmaceuticals.













