Separation of Tryptophan Oxidized Peptides from Their Native Forms
Oxidation of amino acid residues can alter a protein's biological activity, half-life, and immunogenicity.
Oxidation of amino acid residues can alter a protein's biological activity, half-life, and immunogenicity. Peptide maps can be used to detect oxidized peptide fragments. Peptides are commonly separated by RP-HPLC; however, hydrophobic interaction chromatography (HIC) is an alternative technique offering different selectivity.
In this note, we show that native Luteinizing Hormone-Releasing Hormone (LH-RH), a tryptophan (Trp)-containing peptide, is resolved from its forcibly oxidized variants using a ProPac® HIC-10 column (Dionex). The separation of oxidized LH-RH revealed four peaks in addition to the two peaks from the native peptide sample. The native and oxidized LH-RH peaks were identified using mass spectroscopy.
Equipment and Methods
A bioinert liquid chromatography system (Dionex ICS-3000 or UltiMate® 3000 Titanium system) was used to eliminate metal complex formation or redox reactions with peptides. The LH-RH oxidization is described in Dionex Application Note 211 (1).

Figure 1
Results and Discussion
Figure 1a shows the elution of two non-oxidized LH-RH peaks from the ProPac HIC-10 column, at 11 and 18 min (peaks 5 and 6, respectively). Figure 1b shows four additional peaks when the Trp in LH-RH is forcibly oxidized, and the reduced peak areas for peaks 5 and 6 indicate incomplete oxidation. Four different oxidation products, hydroxy-tryptophan (HTRP), N-formylkynurenine (NFK), kynurenine (KYN), and 3-hydroxykynurenine (3OH-KYN) have been described by E.L. Finley et al (2). MS analysis of the oxidized LH-RH shows the presence of four additional major ions (data not shown). MS analysis of the non-oxidized LH-RH showed a single major component with a mass-to-charge ratio equal to that expected for LH-RH. The forced oxidation peaks were identified as peptides having one of two Trp oxidation products, hydroxy-tryptophan or N-formylkynurenine (Table I).
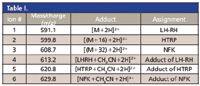
Table I.
Conclusion
The ProPac HIC-10 column can separate oxidized peptides from their native forms showing HIC to be an alternative to RP-HPLC for separation of peptide oxidation variants.
References
(1) Dionex Corporation "Hydrophobic Interaction Chromatography for Separation of Tryptophan and Methionine Oxidized Peptides from Their Native Forms," Application Note 211, LPN 2110, Sunnyvale, CA. In press, 2009.
(2) Finley, E. L., Dillon, J., Crouch, R. K., and Schey, K. L. Protein Science, 1998, 7, 2391–2397.
ProPac and UltiMate are registered trademarks of Dionex Corporation.

Dionex Corporation
1228 Titan Way, P.O. Box 3603, Sunnyvale, CA 94088
tel. (408)737-0700; fax (408)730-9403
Website: www.dionex.com

SEC-MALS of Antibody Therapeutics—A Robust Method for In-Depth Sample Characterization
June 1st 2022Monoclonal antibodies (mAbs) are effective therapeutics for cancers, auto-immune diseases, viral infections, and other diseases. Recent developments in antibody therapeutics aim to add more specific binding regions (bi- and multi-specificity) to increase their effectiveness and/or to downsize the molecule to the specific binding regions (for example, scFv or Fab fragment) to achieve better penetration of the tissue. As the molecule gets more complex, the possible high and low molecular weight (H/LMW) impurities become more complex, too. In order to accurately analyze the various species, more advanced detection than ultraviolet (UV) is required to characterize a mAb sample.















