Separation of Ionic Analytes via Supercritical Fluid Chromatography
The Application Notebook
Supercritical fluid chromatography (SFC) has been practiced for approximately 50 years. SFC on packed columns for both qualitative and quantitative purposes underwent a renaissance in interest at the beginning of the 1990s when limitations of capillary SFC became obvious and important progress in composition gradient techniques for mixed mobile phases was achieved. Even with these instrumental improvements, wide acceptance of the technology was not forthcoming because the perception was that highly polar analytes were not soluble in carbon dioxide and thus were not separable. It is now apparent that the use of additives dramatically extends the range of solute polarity amenable to SFC.
Supercritical fluid chromatography (SFC) has been practiced for approximately 50 years. Early studies used packed columns with varying degrees of success (1) and it would be fair to say that SFC did not generate much interest in the separation science community in this period. In the early 1980s interest in SFC exploded when it was reported that SFC could be easily accomplished with wall-coated open tubular (WCOT) columns similar to the columns currently used for gas chromatography (GC).

The columns differed in two respects: SFC columns had smaller inner diameters and the polymeric stationary phase coating the walls was more extensively cross-linked (2). The emergence of SFC with a mobile phase that exhibited appreciable solvating power relative to helium and hydrogen was primarily promoted by users of GC who saw SFC as a way to increase the limited sample base afforded by GC. This technology, which used pure carbon dioxide, flourished for some time but it had several disadvantages for wide-scale acceptance by the analytical community. Specifically, the technique was traditionally limited to relatively nonpolar compounds because 100% carbon dioxide as the mobile phase exhibited poor solvating power similar to hexane; robust sample injection was not feasible; a wide polarity range of stationary phases was not available; high-quality separations required nearly an hour; gradient carbon dioxide pressure and flow rate were coupled such that flow rate changed as mobile phase pressure changed; passive, fragile restrictors at the column outlet often plugged and the separation had to be aborted; and multigram scale-up of a successful separation was not feasible (3).
Polar Analytes Favor Packed Columns
SFC on packed columns for both qualitative and quantitative purposes underwent a renaissance in interest at the beginning of the 1990s when the limitations of capillary SFC became obvious and important progress in composition gradient techniques for mixed mobile phases was achieved (4). Sophisticated commercial instrumentation allowing independent flow control under both pressure and composition gradient conditions came out in 1992 boosting the development of applications in many major areas of separation science.
By 1997 it was clear that the future of SFC would focus more on the separation of moderately polar analytes with polar-bonded silica-based stationary phases, modified carbon dioxide and spectroscopic detectors. In other words, practical SFC, which affords a bridge between high performance liquid chromatography (HPLC) and GC would be more HPLC-like than GC-like (5). Many of these newer developments were inspired by the work of Dr. Terry Berger who spent more than a decade systematically undoing many of the misconceptions concerning packed column SFC (pcSFC) that existed in the 1980s (6). Some examples of the Berger findings include very long columns with large pressure drops were feasible for SFC; mobile phase carbon dioxide density and solvent strength were disconnected; the effect of mobile phase additives (that is, secondary modifiers) on peak shape and retention were introduced; packed column SFC was shown to be broadly applicable to small drug-like molecules; and quantitative SFC recovery of solutes without conventional cyclone trapping and aerosol generation were demonstrated.
Solubility Versus Activity
Even with these instrumental improvements, wide acceptance of the technology was not forthcoming because the perception was that highly polar analytes were not soluble in a carbon dioxide–based mobile phase and were, therefore, not separable (7). Historically, carbon dioxide has been treated as a nonpolar solvent, primarily because of its low dielectric constant and zero dipole moment. Carbon dioxide has also been described as a quadrupolar solvent because of its significant quadrupole moment.
As far as its microscopic solvent behaviour is concerned, carbon dioxide has the potential to act as both a weak Lewis acid and Lewis base. Strong theoretical and experimental evidence has indicated that carbon dioxide can participate in conventional or nonconventional hydrogen bonding interactions (8). Nevertheless, researchers initially sought to extend the applicability of SFC by either using more polar pure fluids, such as ammonia, sulfur dioxide, nitrous oxide and Freon-23 or by adding a polar organic solvent that is, "modifier") to carbon dioxide to improve the solvating power of the mobile phase (9). Both these experimental routes, however, did not generally lead to successful separations.
In HPLC, the surfaces of almost all packing materials are heterogeneous. Thus, the traditional explanation for lack of analyte elution involves stationary phase "active sites," which usually means the presence of some subset of silanols or metal ions on the traditional bonded-silica packing material. Mobile phase additives in HPLC are very polar substances that are added to the mobile phase at low concentration to improve peak shapes by either covering up, adsorbing on, or reacting with active sites. An alternative approach in HPLC to dealing with active sites involves either silanol endcapping or polymeric coating schemes that are meant to permanently cover active sites, thus eliminating the need for an additive to the mobile phase. All of these strategies have been explored with SFC because the stationary phases used by HPLC and packed column SFC were essentially the same in the beginning.
In the 1990s virtually all peak distortions or nonelution events in pcSFC were attributed either to irreversible adsorption of the very polar analyte to the silica support or (unlike HPLC) to poor solubility of the compound in the modified mobile phase. In pcSFC, endcapping, deactivation and polymer-coated supports, however, enjoyed only limited success (10). The use of additives in conjunction with modifiers — as will be shown later — proved to be as highly successful for SFC as it was for HPLC. Although WCOT columns exhibit much less activity, the elution of even weakly polar analytes was considerably more problematic via this SFC mode because delivery of modified fluids was not experimentally feasible (11). Needless to say, separation of both ionizable and fully ionic analytes with a carbon dioxide–based mobile phase during the early 1990s was thought to be highly unlikely. In other words, SFC provided (at the time) a number of advantages over traditional HPLC, such as speed, practical use of longer columns, a normal-phase retention mechanism and reduced use of organic solvents, but the nature of SFC mobile and stationary phases was thought to not allow the elution of highly polar analytes — much less ionic compounds.
Suffice to say, this situation has drastically changed because experimental approaches using additives have been quite successful. Effective additives are generally too polar to be miscible with carbon dioxide. Instead they are added to the primary modifier and then the modifier plus additive is pumped as a single ternary fluid thereby augmenting the solvating power and flow of pressurized carbon dioxide. In general, ionizable analytes cannot be eluted — or else they are eluted with severely distorted peaks without an appropriate additive in the mobile phase. Today, it is apparent that the use of additives dramatically extends the range of solute polarity amenable to carbon dioxide–based SFC. This review focuses on publications that describe the application of packed column SFC to both ionizable and fully ionic compounds.
Additives Enhance Polar Separations
Berger has suggested that additives perform at least four different functions (12): cover active sites on the solid support; change the polarity of the stationary phase; suppress ionization of the analyte; enhance ion pair formation with the analyte; and raise the polarity and solvating power of the mobile phase. The most obvious role for additives is to change the mobile phase polarity and solvent strength. In this regard, solvatochromic dyes have been used to measure the solvent strength of tertiary mobile phases (13). Small concentrations of additive were sometimes (but not always) found to increase the apparent polarity or solvent strength of the mobile phase. Ion suppression is another rather obvious role for additives, especially if the additive is clearly more acidic (or more basic) than the acidic (or basic) solute.
Figure 1 shows the separation of lovastatin with and without trifluoroacetic acid (TFA) in the mobile phase. TFA is theorized to suppress the ionization of the carboxylic acid group to produce a much sharper peak. In general, the first small addition of additive improves peak shape and sometimes shifts retention. The addition of higher concentrations of the same additive tends to have little further effect. One difference between acidic and basic additives has been noted. Multifunctional acidic additives have been effective in suppressing peak tailing, but multifunctional basic additives have degraded peak shape.

Figure 1
Stationary phase surface coverage by additives is less well defined for conventional columns because the small particle surface is made up of both exposed silica solid support and bonded stationary phase. Surprisingly, carbon dioxide has been shown to strongly adsorb onto column packing. Maximum adsorption of carbon dioxide occurred near the critical temperature and critical pressure (14). Parcher and colleagues have suggested that a thick film of carbon dioxide may act as part of the stationary phase. Modifiers were also shown to strongly adsorb to the stationary phase (15). Because the total amount of adsorbed material increased under these conditions, the adsorption of modifiers was stated to not displace adsorbed carbon dioxide. The quantity of additives that adsorb onto various stationary phases has been measured (16).
Low to moderately polar stationary phases, such as octyl and cyanopropyl, adsorbed only small amounts (0.4–0.6% of a monolayer) of additive. Under the same conditions sulfonic acid and diol columns adsorbed much larger amounts of additive creating surface coverage up to 21% of a monolayer. Finally, certain additives can be very strongly held by the stationary phase thereby changing the stationary phase polarity, vide infra. In this case, additives can cause an increase, a decrease, or no change in solute retention. In these experiments, the sudden introduction of an additive does not result in an immediate change in retention time because the initial process is very similar to a titration with an endpoint.
SFC Equals Normal-Phase HPLC
Chromatographers readily agree that SFC with polar-bonded stationary phases and carbon dioxide–based mobile phases is normal phase chromatography without most of the problems usually associated with classical normal-phase HPLC such as the strong dependence of the retention time on the concentration of small amounts of very polar constituents in the mobile phase. As such, the orthogonality of SFC to reversed-phase LC is an attractive asset for purity assessment (17). Equilibration is very fast, usually requiring flow turnover of little more than three to five column volumes. Composition, pressure and temperature can all be programmed with rapid recovery to initial conditions between analyses.
Solvent characteristics of the fluids used in SFC are not unique to supercritical fluid conditions (12). The characteristics are present irrespective of whether the fluid is defined as a liquid, a dense gas, or supercritical fluid. Many times, the operator cannot tell when the definition (or name) of the mobile phase changes from supercritical fluid to subcritical fluid during a typical separation. As a result, the nomenclature has often been confusing and loosely applied in this area. SFC today usually uses carbon dioxide above or near its critical temperature of 31 °C and critical pressure of 73 bars, combined with an organic modifier such as methanol or ethanol and an additive. The differences in SFC and subcritical fluid chromatography have been overstated in the past. Diffusion coefficient, viscosity, density and solvent strength all show minimal changes when the mobile phase changes from super-to subcritical. When outlet pressure is elevated and internal pressure and temperature are controlled, the resulting techniques are similar and the behavior of conventional GC and HPLC are completely and seamlessly bridged to SFC (18).
SFC has become a powerful tool for both high throughput qualitative and quantitative analyses at various stages of drug discovery and development. Often used in parallel with other separation techniques, SFC has been extensively employed in purity assessment, structure confirmation–elucidation, degradation profiling and formulation assay. The remainder of this review will describe some of the studies where ionic analytes have been chromatographically separated using a carbon dioxide–based mobile phase. The descriptions will draw upon various issues that have been noted earlier in this paper. The primary goal of the manuscript is to demonstrate to the reader that pcSFC is readily applicable to highly polar analytes.
Historic Applications: Ion Suppression Against Ion Pairing
The claimed (but erroneous) chromatographic separation of charged species via SFC dates back to 1988. An advertisement showed a capillary SFC chromatogram of quaternary ammonium salts obtained with carbon dioxide as the mobile phase. After further review by Sandra and David, it was discovered that the quaternary ammonium salts had been converted off-line to amines and the reported separation was of the latter analytes although the title of the offering continued to mislead the reader (19).
In the same year, the separation of five charged enantiomeric 1,2-aminoalcohols as diastereomeric ion pairs using SFC with a cyanopropylsilica stationary phase was reported. N-Benzoxycarbonylglycyl-L-proline, which was dissolved in the acetonitrile modifier along with triethylamine, was employed as the chiral counterion (20). The resulting solution was mixed with liquid carbon dioxide in a 1:4 (v/v) ratio. Triethylamine served as the competing ion as it became protonated by the carbon dioxide phase. The retention and separation efficiency was stated to be controlled by pressure and temperature as well as by the concentration and nature of the ionic mobile phase components. Variation of the nature and concentration of the counterion and the competing ion was predicted to make the system highly versatile.
These ion-pairing principles were further tested via SFC two years later (21). Successful elution of propanolol hydrochloride from a cyanopropylsilica packed column with 25 mM sodium heptane sulfonate in methanol-modified carbon dioxide was reported. Without sulfonate in the mobile phase, the analyte was not eluted. The influence of dimethyloctylamine, tributylamine, and citric acid on chromatographic selectivity was studied. A prime limitation of ion-pair SFC was found to be the solubility of ion-pairing agent in the carbon dioxide–modifier mixture. As a rule of thumb, the paper suggested that the best choice of initial conditions when starting to optimize the separation of ionizable compounds was to use a diol phase, tributylamine, and acetate ion in methanol as the ion-pairing agent.
Much later an assay of berberine and palmatine in Phellodendri Cortex was established using ion-pairing SFC on-line coupled with ion-pair supercritical fluid extraction (SFE) (22). Dioctyl sodium sulfosuccinate in methanol (100 mM) served as the ion-pairing agent for both the SFE and the SFC experiments both of which were completed in approximately 20 min. A silica gel column was used as an SFE trapping and SFC separation column. The modifier was 10% (v/v).
The need for an additive to facilitate separation of ionizable analytes was challenged years later by Geiser (23). Enantioseparation of ionizable amine hydrochloride salts and free acids without the use of amine or acid additives was reported. Examples were shown for racemic mixtures of three amine salts (propranolol, thioidazine, and tramadol) and a carboxylic acid (flurbiprofen). Tandem ultraviolet and polarimetric detection in conjunction with a Chiralpak AD-H chiral stationary phase using methanol-modified carbon dioxide was used. A wide dynamic range, suitable for preparative chromatography, was demonstrated for column injections of 0.15–6 mg rac-flurbiprofen.
Although the authors did not comment on this aspect, the study is subject to criticism in that the free base and free acid may be the actual species being separated rather than the salt as claimed. For example, the amine salts investigated are derived from either secondary or tertiary amines and in solution they may exist in equilibrium with the free amine. In other words, good evidence for the separation of the actual amine salt appears to be missing. As far as the acid, flurbiprofen, is concerned, the inherent acidity of carbon dioxide should ensure the predominance of the neutral acid form in the mobile phase. As before, proof for the separation of the conjugate base of flurbiprofen appears to be missing from the report. It should be noted, however, that for many acidic analytes the acidity of carbon dioxide is not sufficient to totally suppress ionization. For example, SFC of a variety of acidic polyphenols has been successfully studied with several acid additives such as citric acid and trifluoroacetic acid (24). While certain polyphenols eluted with sharp peaks without additive, others, which were more strongly acidic, required additive.
Uncertainty regarding elution of the free base versus the amine salt is prevalent in the early study by Gyllenhaal and colleagues (25). Metoprolol tartrate, metoprolol succincate, and hydrochloride analogues were initially dissolved in dichloromethane. SFC was then performed on both a diol-silica or aminopropyl-silica column with triethylamine (TEA) or TEA mixed with acetic acid. Given these conditions, separation of the free base was most likely occurring.
Gyllenhaal and colleagues have also investigated the effects of binary additives in the mobile phase for separation of metoprolol and related amino-alcohols (26). Use of ethanesulfonic acid (ESA) and triethylamine as binary additives to methanol modifier and a diol column showed that when the additives were discontinued their positive effect disappeared virtually instantaneously. Standard conditions used were 10% methanol, containing 24 mM of acid and 18 mM of TEA, in carbon dioxide with a flow-rate of 1.5 mL/min. The column dimensions were 125 mm × 4 mm and kept at 40 °C with a back pressure of 150 bar. The effects on selectivity were stronger with trifluoroacetic acid than ethanesulfonic acid. The described approach was thought to offer a way to tune the selectivity of SFC systems when amines are analyzed without the need to change the stationary phase of the chromatographic system. Subsequently, these same workers have reported the separation of these analytes without an amine and basic additive from both an aminopropyl-silica and an ethylpyridine-silica column (27).
Recent Applications: Ion Suppression Versus Ion Pairing
Pinkston and colleagues brought new thinking to this area recently when they showed that low levels of volatile ammonium salts as mobile phase additives allow the elution of polar and even ionic organic materials from a Deltabond cyanopropyl-bonded silica column (28). In contrast to other common additives, the volatile ammonium salts were compatible with mass spectrometric detection. The SFC-UV chromatograms of reserpine standard with methanol and with 1.1 mM ammonium acetate in methanol are shown in Figure 2.

Figure 2
Two mechanisms depending on the presence or absence of ammonium salt were suggested to have the greatest influence on retention in the carbon dioxide–methanol–ammonium salt–Deltabond cyano system. The salt effect was stated to be a result of either ion pairing, deactivation of the silica surface, or both. The effect of ammonium acetate additive in the mobile phase for the elution of seven pharmaceuticals is shown in Figure 3. None of these analytes are eluted with only a methanol modifier.

Figure 3
In a closely related study, the effect of various ionic additives on the elution of ionic compounds such as 4-dodecylbenzene sulfonate and sodium 4-octylbenzene sulfonate has been determined (29). The additives were lithium acetate, ammonium acetate, tetramethylammonium acetate, tetrabutylammonium acetate, and ammonium chloride dissolved in methanol. Three stationary phases with different degrees of deactivation were considered: conventional cyanopropyl, Deltabond cyanopropyl, and bare silica. Each sulfonate salt was successfully eluted using all the additives with good peak shape under isocratic–isobaric–isothermal conditions. The column memory effect created via strong interaction of the additive with silanol sites for sodium p-toluene sulfonate on a silica column with tetrabutylammonium acetate as additive is illustrated by the series of chromatograms in Figure 4. Different additives yielded different retention times and in some cases different peak shapes.
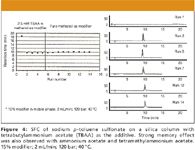
Figure 4
The presence of the additive was crucial concerning elution of the sulfonate salts. Solid state silicon-29 nuclear magnetic resonance spectroscopy suggested that ammonium ions deactivated available silanol sites on both the Deltabond phase and the bare silica (30). Ammonium ion was also speculated to interact with cyano-sites on the bonded phase. Computational calculations concerning the charge distribution on various ammonium salts were performed in an effort to explain the elution behavior of the sulfonate salts. The acetate counterion was thought to facilitate elution of the anionic sulfonates from the positively charged stationary phase in a pseudo ion-exchange mechanism. On the bare silica where abundant silanol sites are present, modification of the stationary phases was thought to be the dominant effect. Ammonium ion–induced dipole interaction with the cyano function may be dominant with the more deactivated Deltabond cyano phase.
Elution of cationic species such as amine salts in SFC is more difficult to prove unless the amine salt is quaternary (that is, NR4+ X- ) (31). Otherwise, equilibrium with the neutral form may give rise to elution of only the neutral or a mixture of the conjugate pair. In this regard, the successful elution of two quaternary amine salts from a Deltabond cyano-bonded silica column with the addition of a sodium alkylsulfonate to the methanol-modified carbon dioxide–based mobile phase has been reported. An ion pairing interaction between the positively charged analyte and the anionic part of the alkylsulfonate additive was proposed. This study demonstrated the reciprocity of ion-pair SFC in that if amine salts are good additives for elution of sulfonate salts, then sulfonate salts should be good additives for elution of amine salts. This observation, however, does not speak completely to ion-pair SFC, but rather to additive deactivation of the stationary phase because Hoke and colleagues (32) have shown via packed column SFC–tandem mass spectrometry (MS–MS) that cetylpyridinium chloride in saliva is readily eluted from a 5 cm Deltabond cyano column with ammonium acetate additive in the mobile phase in approximately 12 s with a retention factor of 2.
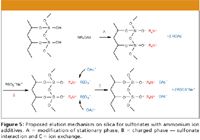
Figure 5
In some cases, workers have discovered that conversion of the neutral analyte into an ion actually enhances efficient chromatographic separation by favoring an ion-pairing mechanism. Stringham has reported that incorporation of a strong acid such as ESA in both the sample solvent and mobile phase modifier gives a dramatic improvement in the separation of chiral basic compounds (33). Removal of the acid from either the sample solvent or the mobile phase resulted in loss of the separation. The mechanism of separation appeared to involve an intact salt pair formed between the protonated basic compound and the conjugate base of ESA. Methanesulfonic acid consistently gave shorter retention times than ESA. With camphorsulfonic acid, selectivity was consistently lower than that observed with ESA. TFA in place of ESA gave either broad or absent peaks.
Blackwell and Stringham succeeded to elute peptides such as bradykinine and oxytocin from a polymeric column using eptadecafluorooctanesulfonic acid as an additive in methanol with carbon dioxide from a divinylbenzene polymeric (that is, 500 Å) column (34). Ion pair effects were discussed as being responsible for the enhanced elution compared with methanesulfonic acid and TFA as additives. The potassium salt of heptadecafluorooctanesulfonic acid as an additive failed to elute any of the polypeptides. The role of the modifier was found to be secondary to that of the mobile phase additive. As progressively stronger acidic mobile phase additives were used, the peak profiles of the various polypeptides improved and retention decreased.
A more recent report concerns the separation of polypeptides containing a variety of acidic and basic residues with up to 40 mers via packed column SFC–MS (35). TFA was employed as the additive in a carbon dioxide–methanol mobile phase (which was compatible with MS detection) to both suppress deprotonation of peptide carboxylic acid groups and to protonate peptide amino groups. A 2-ethylpyridine-silica column was used for the study.
Figure 6 shows mass chromatograms of five polypeptides (angiotensin I, angiotensin II, angiotensin III, urotensin II, and sauvagine) with various mobile phase modifier–additive combinations. All three angiotensins gave the sharpest peak shapes with 13 mM TFA in methanol as modifier. Urotensin II also was eluted fastest and provided the best peak shape with 13 mM TFA in methanol. With the salt of TFA (that is, ATFA), the peptide was retained strongly. Sauvagine contains over 40 residues, having a molecular mass of 4500 Da including four basic amino acid groups and seven acidic amino acid groups. Only when 13 mM TFA in methanol was used as modifier was sauvagine eluted with a sharp symmetrical peak shape. The mechanism of separation is not known. No doubt TFA plays several important roles such as both protonation of the analyte and the ethylpyridine stationary phase. It, however, is clear that polar, high molecular weight, protonated polypeptides have been chromatographed employing some form of ion-pair (or ion-exchange) SFC–MS.
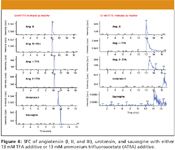
Figure 6
Packed column SFC has experienced tremendous growth as an analytical and preparative technique over the past 20 years (36). Unlike GC and HPLC, where various retention models have been extensively developed for years, SFC suffers from the lack of systematic studies on retention mechanisms and useful models for solute retention. Wu has discussed three different retention models: empirical models, thermodynamic models, and extrathermodynamic (that is, linear free energy relationships) models (37). The ion pairing principle in SFC may not match any of these general retention mechanisms. Nevertheless, SFC of highly polar analytes appears to have been demonstrated on numerous occasions over the past 40 years. As more separation scientists grasp the true benefits of SFC, new applications are expected with enhanced mechanistic and thermodynamic understanding.
Acknowledgment
The assistance of Negin Nazem during the preparation of this manuscript is gratefully appreciated.
Larry T. Taylor is Emeritus Professor of Chemistry at Virginia Tech, Blacksburg, Virginia, USA. He serves on the editorial boards for Journal of Chromatographic Science, Chromatographia, and Journal of Supercritical Fluids. He teaches short courses on SFC and SFE. Approximately 200 peer-reviewed publications dealing with SFC and SFE have been published. He Chaired the Scientific Committee for both SFC 2008 and SFC 2009 international conferences.
References
(1) S.T. Sie and G.W.A. Rijnders, Sep. Sci. 2, 699–727 (1967).
(2) M. Novotny et al., Anal. Chem. 53, 407A–414A (1981).
(3) P.R. Griffiths, Anal.Chem. 60, 593A–597A (1988).
(4) L.T. Taylor, J. Chromatogr. Sci. 28, 357–366 (1990).
(5) L.T. Taylor, J. Chromatogr. Sci. 35, 374–382 (1997).
(6) T.A. Berger, Practical Advantages of Packed Column Supercritical Fluid Chromatography in Supporting Combinatorial Chemistry in Unified Chromatography, ed by J.F. Parcher and T.L. Chester,. ACS Sym. Series, 748, 203–233 (2000).
(7) P.J. Schoenmakers and L.G.M. Uunk, European Chromatogr. News 1, 14–20 (1987).
(8) P. Raveendran, Y. Ikushima, and S.L. Wallen, Acc. Chem. Res. 38, 478–485 (2005).
(9) T.A. Berger and W.H. Wilson, J. Pharm. Sci. 84, 489–492 (1995).
(10) M. Ashraf-Khorassani, L.T. Taylor, and R.A. Henry, Anal. Chem. 60, 1529–1533 (1988).
(11) M.C. Henry and C.R. Yonker, Anal. Chem. 78, 3909–3915 (2006).
(12) T.A. Berger, Packed Column SFC (Royal Society of Chemistry, Letchworth, UK, 1995).
(13) T.A. Berger and J.F. Deye, Use of Solvatochromic Dyes to Correlate Mobile Phase Solvent Strength to Chromatographic Retention in Supercritical Fluid Chromatography, ACS Sym. Ser. 488, 132–142 (1992).
(14) J.R. Strubinger, H. Song, and J.F. Parcher, Anal. Chem. 63, 98–103 (1991).
(15) J.R. Strubinger, H. Song, and J.F. Parcher, Anal. Chem. 63, 104–108 (1991).
(16) T.A. Berger and J.F. Deye, J. Chromatogr. 547, 377–392 (1991).
(17) F.J. Akin, Curr. Pharm. Anal. 3, 53–70 (2007).
(18) T.L. Chester and J.D. Pinkston, Anal. Chem. 76, 4606–4613 (2004).
(19) F. David and P. Sandra, J. High Resol. Chromatogr. 11, 897–898 (1988).
(20) W. Steuer, M. Schindler, G. Schill, and F. Erni, J. Chromatogr., A 447, 287–296 (1988).
(21 W. Steuer, J. Baumann, and F. Erni, J. Chromatogr., A 500, 469–479 (1990).
(22) K. Suto et al., J. Chromatogr., A 786, 371–376 (1997).
(23) F. Geiser and R. Shah, Chirality 16, 263–266 (2004).
(24) M. Kamangerpour. Chromatographia 55, 417–421 (2002).
(25) O. Gyllenhaal and J. Vessman, J. Chromatogr., A 839, 141–148 (1999).
(26) O. Gyllenhaal, L. Edstrom, and B-A. Persson, J. Chromatogr., A 1134, 305–310 (2006).
(27) J. Lundren, J. Chromatogr., A 1154, 360–367 (2007).
(28) J.D. Pinkston, D.T. Stanton, and D. Wen, J. Sep. Sci. 27, 115–123 (2004).
(29) J. Zheng et al., J. Chromatogr., A 1082, 220–229 (2005).
(30) J. Zheng et al., J. Chromatogr., A 1090, 155–164 (2005).
(31) J. Zheng et al., Chromatographia 63, 267–276 (2006).
(32) J.A. Tomlinson, T.H. Eichold, R.E. Barron, and S.H. Hoke, private communication.
(33) R.W., Stringham, J. Chromatogr. A 1070, 1630–70 (2005).
(34) J.A. Blackwell and R.W. Stringham, J. High Resol. Chromatogr. 22, 74–78 (1999).
(35) J. Zheng et al., Anal. Chem. 78, 1535–1545 (2006).
(36) L.T. Taylor, J. Supercrit. Fluids 47, 566–573 (2008).
(37) Y. Wu, J. Liq. Chromatogr. Rel. Tech. 27, 1203–1236 (2004).

New Method Explored for the Detection of CECs in Crops Irrigated with Contaminated Water
April 30th 2025This new study presents a validated QuEChERS–LC-MS/MS method for detecting eight persistent, mobile, and toxic substances in escarole, tomatoes, and tomato leaves irrigated with contaminated water.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)


















