Comparison of Liquid-Liquid Extraction (LLE) and Supported Liquid Extraction (SLE): Equivalent Limits of Quantitation with Smaller Sample Volumes
Traditional liquid-liquid extraction (LLE) can provide very clean extract. However, in many cases lower recoveries, labor-intensive liquid handling issues, and difficulty with automation can limit the success of LLE in sample preparation.
Traditional liquid-liquid extraction (LLE) can provide very clean extract. However, in many cases lower recoveries, labor-intensive liquid handling issues, and difficulty with automation can limit the success of LLE in sample preparation. Supported Liquid Extraction (SLE), a 96-well high-throughput technique is superior to traditional LLE because the extraction interface occurs between a buffered sample, which is absorbed onto an inert solid support and a water immiscible solvent. The high surface area of the material provides excellent extraction efficiency, while alleviating many of the liquid handling issues associated with traditional LLE. This application note compares ISOLUTE SLE+ with traditional LLE in terms of recoveries and demonstrates equivalent limits of quantitation with smaller sample volumes using the supported liquid extraction (SLE+) approach.
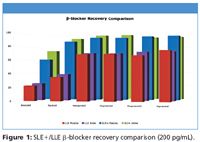
Figure 1
Sample Preparation Procedure
Supported Liquid Extraction Procedure Plate: ISOLUTE SLE+ 400 Supported Liquid Extraction Plate, part number 820-0400-P01.
Sample Pre-treatment:
Acidic analytes (NSAIDs): Plasma (200 μL) pre-treated 1:1 v/v with 1% formic acid aq.
Basic analytes (β-blockers): Plasma (200 μL) pre-treated 1:1 v/v with 0.5 M ammonium hydroxide.
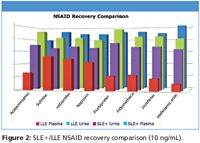
Figure 2
Sample Application: The pre-treated plasma (total 400 μL) was loaded onto the plate, a pulse of vacuum applied to initiate flow and the samples left to absorb for 5 min.
Analyte Extraction: Addition of 2 × 900 μL of either MTBE (NSAIDs) or EtOAc (β-blockers).

Figure 3
Liquid-liquid Extraction Procedure
Plasma (500 μL) was pre-treated 1:1 v/v with: 1% formic acid aq and extracted with 1.8 mL of MTBE (NSAIDs); or 0.5 M ammonium hydroxide and extracted with EtOAc (β-blockers). The layers were left to separate and the organic aliquot removed.
Post Extraction: The extract was evaporated to dryness and the analytes reconstituted in 500 μL of appropriate H2O/MeOH mixtures prior to analysis.

Figure 4
HPLC Conditions
Instrument: Waters 2795 Liquid Handling System (Waters Assoc., Milford, MA, USA)
Column: Zorbax Eclipse XDB C18 3.5 μm analytical column (100 × 2.1 mm id, 3.5 μm) (Agilent Technologies, Berkshire, UK).
Guard Column: C8 guard column (Agilent Technologies, Berkshire, UK).
Mobile Phase: 0.1% formic acid aq and MeCN (acetonitrile) at a flow rate of 0.25 mL/minute using various gradients.
Injection Volume: 15–25 μL
Temperature: Ambient
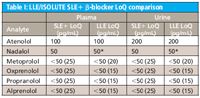
Table I: LLE/ISOLUTE SLE+ β-blocker LoQ comparison
Mass Spectrometry
Instrument: Ultima Pt triple quadrupole mass spectrometer (Waters Assoc., Manchester, UK) equipped with an electrospray interface for mass analysis. Positive and negative ions were acquired in the multiple reaction monitoring mode (MRM). All β-blockers were analysed in positive ion mode, whereas, the NSAIDs required both positive and negative MRM transitions.
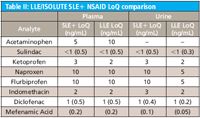
Table II: LLE/ISOLUTE SLE+ NSAID LoQ comparison
Desolvation Temperature: 350 °C
Ion Source Temperature: 100 °C
Collision Gas Pressure: 2.4 × 10-3 mbar
Results
Figures 1 and 2 show SLE+ and LLE recovery data for the β-blockers and NSAID's, respectively. This data is based on the recovery compared to blank plasma sample fortified post extraction at the same concentration. Figures 3 and 4 show spiked sample response against a standard at the same concentration for the β-blockers and NSAIDs, respectively. This data takes into account the recovery and suppression observed using the two techniques. Tables I and II show limits of quantitation observed for the β-blockers and NSAIDs, respectively. Some analyte LoQs were lower than the lowest level extracted and as a result the level was estimated based on the lowest level signal-to-noise.
Conclusions
- Isolute SLE+ provides higher recoveries and better spiked signal response compared to LLE for all analytes.
- Isolute SLE+ shows similar LoQs for a number of the analytes screened when extracting 200 μL compared to 500 μL plasma extractions using LLE. This illustrates that lower sample volumes can be used with supported liquid extraction as compared to traditional LLE.
- Lower LoQs were possible for some analytes with LLE. However, the differences were not significant and similar levels could be obtained using a higher plasma to buffer ratio with ISOLUTE SLE+.

Biotage AB
Kungsgatan 76, SE-753 18, Uppsala, Sweden
tel. (146)18-56-56-10; fax (46)18-56-57-05
Email:
order@eu.biotage.com
;
Website:
www.biotage.com

SEC-MALS of Antibody Therapeutics—A Robust Method for In-Depth Sample Characterization
June 1st 2022Monoclonal antibodies (mAbs) are effective therapeutics for cancers, auto-immune diseases, viral infections, and other diseases. Recent developments in antibody therapeutics aim to add more specific binding regions (bi- and multi-specificity) to increase their effectiveness and/or to downsize the molecule to the specific binding regions (for example, scFv or Fab fragment) to achieve better penetration of the tissue. As the molecule gets more complex, the possible high and low molecular weight (H/LMW) impurities become more complex, too. In order to accurately analyze the various species, more advanced detection than ultraviolet (UV) is required to characterize a mAb sample.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)














