Selective Detection with UV Spectroscopy in Gas Chromatography
Featured Articles
Using UV spectroscopy as a selective detection approach in gas chromatography (GC) has been demonstrated here with a diode array detector (DAD). The benefits of using an UV detector in GC were realized through effective thermal management and inertness improvement. The approach deployed in this study was proven to be effective for either tandem or parallel detection and has the potential to be used in chromatographic separations that require stringent, narrow bandwidths, such as comprehensive two-dimensional gas chromatography (GCxGC).
One-dimensional gas chromatography (GC) with high-resolution capillary column technology is a first-line approach for volatile organic compound (VOC) analyses. The high-resolution capillary column technology has achieved much, yet more capability for separation is desired. Challenges continue to exist in several areas, especially when dealing with increasingly complex matrices (1,2). One of the approaches that can be used to address the excessive time required for separation caused by a lack of column selectivity is selective detection. This analytical strategy can substantially ease the burden of separation imposed on the separation column and achieve higher sample throughput. By design, selective detection also offers improved sensitivity for targeted compounds. This feature can be quite beneficial because the improved sensitivity can reduce or eliminate the need for time-consuming sample preconcentration. The strategy of distributing the separation power requirements amongst the separation and detection steps is critical for the continued development of GC as a highly capable and sustainable analytical technique.
UV Detection for GC
Among the selective detection schemes, UV spectrophotometry is a viable option because it has several essential capabilities. First, UV detection operates without the requirement for reduced pressure environments. Ambient pressure operation can lead to fast analytical system start-up and stabilization. Second, many organic compounds amenable to chromatographic separations show an absorption spectrum in the UV-vis regions. Therefore, successful hyphenation of UV detection to GC is indicated for both qualitative and quantitative analysis. Third, the nondestructive nature of UV detection is amenable for serial and multidetection approaches. Also, gas-phase spectra can be generated, which can be helpful for provisional structural elucidation, especially when UV spectrophotometry is deployed as an adjuvant technique with mass spectrometry (MS). Finally, GC-UV has respectable analyte detectability with detection limits in the nanogram range, which is compatible with many current requirements.
Since the early 1960s, efforts to employ UV detection with GC have been explored and reported in the literature by technology pioneers and early adopters (3–15). With UV detection, increased sensitivity and selectivity can be achieved for many classes of compounds with chromophores. Recently, new endeavors to expand this field of practice by exploiting the vacuum UV (VUV) region in addition to the traditional UV range with a dedicated analytical detection system have also been reported (16–18). Some of the barriers to UV being adopted as a detection technique include the performance and reliability of the light source and the successful commercialization of alternative technologies such as benchtop MS. Our goal is to advance the use of UV as a selective gas-phase detector through the interfacing of a current diode array detector (DAD) initially designed for liquid chromatography (LC) to a GC instrument. The detector was based on a cartridge cell design with optofluidic waveguides that improve light transmission with full spectral detection.
Conceptually, DAD can be highly advantageous for use with narrow chromatographic peaks encountered in GC with several positive attributes, such as a high data-sampling rate of up to 240 Hz and the capability to track up to eight wavelengths simultaneously. The nondestructive nature of DAD permits the incorporation of a second detector in series, which provides information-rich chromatography (demonstrated later in this article), without incurring extra analytical time nor column effluent splitting, which can reduce overall detectability. DAD brings UV-vis spectroscopy (190–640 nm) onto the capillary GC timescale, enabling spectrophotometric detection of analytes with peak widths as narrow as 450 ms.
Critical modifications and improvements to the existing detector design and performance were made to overcome known challenges commonly encountered in GC. Specifically, the detector requires an effective strategy for heat management and inertness improvement. In GC, heat management is essential for intercomponent integration and to safeguard optimal individual chromatographic component performance. A novel heated cell was constructed and implemented to preserve the excellent chromatography obtained with high-resolution capillary columns. Concepts and strategies of heating the detector cell were investigated to improve reliability and to simplify thermal process control. Heating the detector cell typically involves using multiple components such as a heater, a feedback-and-control mechanism, and a safety device to prevent overheating. Among many options explored, the discovery and implementation of a positive temperature coefficient (PTC) thermistor as a heater was the most efficient (19). The use of PTC heaters offers substantial benefits such as energy efficiency, protection against over heating, size, and scalability.
Achieving a highly inert sample flow path is another essential criterion (20–22). Although analyte flow path inertness is not a central issue for other chromatographic techniques because of the liquid mobile phase, in GC, it is imperative to achieve the most inert flow path possible. Effective deactivation schemes were applied to ensure analytes are not absorbed or adsorbed in the optical cell employed by the DAD.
By incorporating positive temperature coefficient technology into a commercial DAD detector and through successful passivation of the optical cell, the detector was made suitable for coupling with GC (19). The detection strategy can be incorporated into a standard GC system configuration, including one-dimensional GC and multidimensional GC, including GCxGC. Two chromatographic configurations with polarized separation power are shown here to illustrate the versatility of the detection strategy.
UV Detection in GC-DAD-FID
The nondestructive feature of the DAD allows a flame ionization detector (FID) to be incorporated so that it can provide information-rich analyses. This arrangement delivers near-simultaneous selective and universal detection without increasing analytical time and recourse to column flow splitting. Figure 1a shows a chromatogram of a mixed standard of 1000 ppm (v/v) each of alkanes from C1 to C6 and carbon disulfide in air with a DAD in tandem with an FID. A suppressed response of more than 200 times was noted for carbon disulfide by FID compared to hexane with the same detection means. This lack of response is because of the limited formation of CHO+ by carbon disulfide in a hydrogen flame.
FIGURE 1: Chromatogram of a mixed standard of 1000 ppm (v/v) each of alkanes and carbon disulfide in air by GC-DAD-FID on the FID channel. (b) Overlay of chromatograms of a mixed standard of 1000 ppm (v/v) each of alkanes and carbon disulfide in air by GC-DAD-FID on the DAD channel at 191 and 214 nm. Conditions for (a) and (b) are as described in reference (21,22).
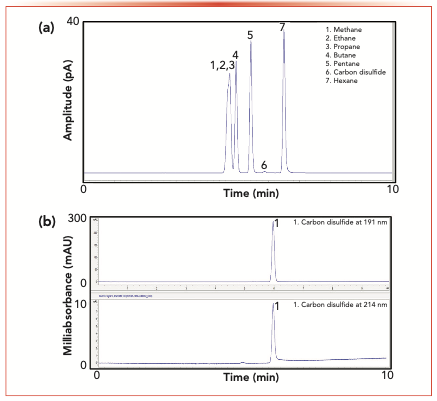
Meanwhile, only the presence of carbon disulfide was detected by the DAD at two simultaneously monitored wavelengths of 191 nm and 214 nm, as shown in Figure 1b. No response was observed for the alkanes on both wavelengths. The selectivity gained can be quite advantageous to shorten the analytical time required if monitoring of carbon disulfide in a hydrocarbon matrix is required. Using the DAD, a substantial improvement in signal detectability for carbon disulfide of at least one order of magnitude was realized as compared to the response generated using the FID (22). With the capability to conduct simultaneous multiwavelength monitoring and with this combined detection strategy, extra analytical information on the chemicals can be obtained to enhance detectability and improve the accuracy of structural elucidation.
UV Detection in GC×GC
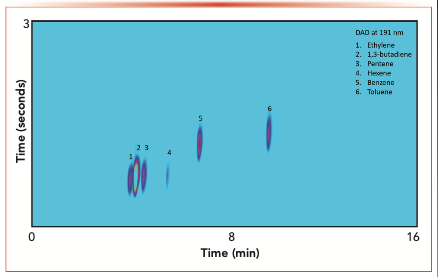
We have demonstrated the potential suitability of the strategy with chromatographic techniques having the most stringent requirements on band broadening, such as high-resolution gas chromatography with columns having an internal diameter of 150 μm and particularly with GC×GC. Figure 2 shows a color plot of a mixed standard comprising alkenes, 1,3-butadiene, benzene, and toluene in nitrogen by GC×GC with a DAD operating at 191 nm in tandem with an FID. An average peak width at a half-height (PWh) of 450 ms was achieved without extensive system optimization. When combined with a selective and nondestructive detector, such as the DAD, and a universal organic carbon compound detector, such as the FID, the enhanced separation power of GCxGC provides a practical approach for the analyst with additional insight into the chemical composition of the sample.
FIGURE 2: Color plot of a mixed standard of alkenes, 1,3-butadiene, and aromatics in nitrogen using GC×GC. Conditions are as described in reference (21,23).
The adoption rate of UV detection for GC by practitioners has been slow to moderate. Plausible barriers that may have limited development include challenges associated with constructing suitable detector–GC interfaces, the challenges encountered with light sources like additional time to reach stability and the regressive decay of light intensity over time, and the design of the flow cell to achieve new limits of detection.
With the advent of advanced electronics, effective heating management, novel high power, and a light source with selective wavelength, UV detection has great potential as a highly reliable and effective approach for gas chromatography.
Acknowledgments
Drs. Wayde Konze, Tonya Stockman, and Matthias Pursch of the Dow Chemical Company are acknowledged for their support. Dr. Grace Xiuhan Yang, also of Dow Chemical, is acknowledged for her help in preparing the manuscript. Agilent Technologies is acknowledged for providing a 7117B diode array detector for the work. This project is partially supported by the Dow 2020 and 2021 Analytical Science’s Capability Development Project Fund.
References
(1) W. Jennings, Applications of Gas Capillary Chromatography (Marcel Dekker, New York, New York, 1981).
(2) W. Jennings, E. Mittlefehldt, and P. Stremple, Analytical Gas Chromatography (Academic Press, New York, New York, 2006).
(3) W. Kaye, Anal. Chem. 34, 287–293 (1996).
(4) D.J. Bornhop, L. Hlousek, M. Hackett, H. Wang, and G.C. Miller, Rev. Sci. Instrum. 63, 191–201 (1992).
(5) D.J. Bornhop and G. Verga, Trend Anal. Chem. 11, 194–198 (1992).
(6) D.J. Bornhop and J.G. Wangsgaard, J. High Resolut. Chrom. 14, 344–347 (1991).
(7) M. Hackett, H. Wang, G.C. Miller, and D.J. Bornhop, J. Chromatogr. A 695, 243–257 (1995).
(8) V. Lagesson and L. Lagesson-Andrasko, Arrangement at a gas flow through cell for spectrophotometric analysis of chemical compounds, U.S. Patent 4,668,091A, May 26, 1987.
(9) V. Lagesson and J.M. Newman, Anal. Chem. 61, 1249–1252 (1989).
(10) L. Lagesson-Andrasko, V. Lagesson, and J. Andrasko, Anal. Chem. 70, 819–826 (1998).
(11) H.V. Lagesson, L. Lagesson-Andrasko, J. Andrasko, and F.J. Baco, J. Chromatogr. A 867, 187–206 (2000).
(12) T. Cedron-Fernandez, C. Saenz-Barrio, S. Cabredo-Pinillos, and I. Sanz-Vicente, Talanta 57, 555–563 (2002).
(13) J. Merritt, F. Comendant, S.T. Abrams, and V.N. Smith, Anal. Chem. 35, 1461–1464 (1963).
(14) M. Novotny, F.J. Schwende, M.J. Hartigan, and J.E. Purcell, Anal. Chem. 52, 736–740 (1980).
(15) M. Kube, M. Tierney, and D.M. Lubman, Anal. Chim. Acta 171, 375–379 (1985).
(16) K.A. Schug, I. Sawicki, D.D. Carlton, H. Fan, H.M. McNair, J.P. Nimmo, P. Kroll, J. Smuts, P. Walsh, and D. Harrison, Anal. Chem. 86, 8329–8335 (2014).
(17) L. Bai, J. Smuts, P. Walsh, H. Fan, Z. Hildenbrand, D. Wetz, and K.A. Schug, J. Chromatogr. A 1388, 244–250 (2015).
(18) H. Fan, J. Smuts, L. Bai, P. Walsh, D.W. Armstrong, and K.A. Schug, Food Chem. 194, 265–271 (2016).
(19) R. Gras, J. Luong, M. Pursch, and R. Shellie, Anal. Chem. 90, 6426–6430 (2018).
(20) R. Gras, J. Luong, and R.A. Shellie, Anal. Chem. 87, 11429–11432 (2015).
(21) R. Gras, J. Luong, and R.A. Shellie, J. Chromatogr. A 1500, 153–159 (2017).
(22) R. Gras, J. Luong, and R.A. Shellie, Anal. Methods 9, 3908–3913 (2017).
(23) X. Guan, J. Luong, Z. Yu, and H. Jiang, Anal. Chem. 92, 6251–6256 (2020).
Ronda Gras and Jim Luong are with Dow Chemical Canada ULC in Fort Saskatchewan, Alberta, Canada, and with the Australian Centre for Research on Separation Science, in Hobart, Tasmania, Australia. Robert A. Shellie is with the CASS Food Research Centre in the School of Exercise and Nutrition Sciences at Deakin University in Burwood, Australia. Direct correspondence to Jim Luong at luong@dow.com.

University of Rouen-Normandy Scientists Explore Eco-Friendly Sampling Approach for GC-HRMS
April 17th 2025Root exudates—substances secreted by living plant roots—are challenging to sample, as they are typically extracted using artificial devices and can vary widely in both quantity and composition across plant species.
Sorbonne Researchers Develop Miniaturized GC Detector for VOC Analysis
April 16th 2025A team of scientists from the Paris university developed and optimized MAVERIC, a miniaturized and autonomous gas chromatography (GC) system coupled to a nano-gravimetric detector (NGD) based on a NEMS (nano-electromechanical-system) resonator.
Miniaturized GC–MS Method for BVOC Analysis of Spanish Trees
April 16th 2025University of Valladolid scientists used a miniaturized method for analyzing biogenic volatile organic compounds (BVOCs) emitted by tree species, using headspace solid-phase microextraction coupled with gas chromatography and quadrupole time-of-flight mass spectrometry (HS-SPME-GC–QTOF-MS) has been developed.












