Remember Harold McNair: Three Fundamental Areas of Understanding in Gas Chromatography
On June 27, 2021, Harold Monroe McNair passed away peacefully in his home, surrounded by his family. Harold’s career as a chromatographer spanned over 60 years, from his 1959 authoring of the first gas chromatography (GC) doctoral thesis in the United States on stationary phase chemistry to the first edition of his classic book, Basic Gas Chromatography, in 1964, to a 2019 journal article on the diet of post-weaning heifers (cows). McNair’s remarkable career and writings spanned the entire breadth of chromatography and separation science. Using McNair’s early writings and works as a guide, I explore three fundamental areas of understanding in GC, seeing ideas about problems that still challenge gas chromatographers today. With McNair, we explore stationary phase chemistry, computers in chromatography, and liquid chromatography (LC) compared to GC.
Harold McNair had a remarkable 60-year career as a chromatographer, and his writings spanned the entire breadth of chromatography and separation science. By looking back at some of his key early writings, we have an opportunity to explore three key areas in gas chromatography (GC), which are still relevant today: stationary-phase chemistry, chromatography and computers, and liquid chromatography (LC) versus GC.
Understanding Stationary-Phase Chemistry
Harold McNair’s doctoral dissertation, presented to the faculty of Purdue University in 1959, entitled “Efficiency of Solvents in Gas Chromatography,” was the first doctoral thesis on GC in the United States (1). The original thesis is freely available by open access through the university and ProQuest. The thesis title alone offers interesting historical insight because the thesis was written prior to the definition of many of the currently used terms in chromatography (2). When we think of efficiency today, we mostly discuss peak widths and theoretical plates. In 1959, McNair was discussing retentiveness and selectivity. When we think of solvents today, we would likely think of sample preparation or dilution solvents; McNair was discussing the stationary phase. Translated to the terminology of today, this title might read something like: “Retentiveness of stationary phases in gas chromatography.” McNair performed the first systematic study of retention and polarity of stationary phases in GC, which is a topic that we are still debating and discussing today.
McNair’s thesis also provides insight into how gas chromatographs were constructed back in the 1950s before commercial instruments were available. Figure 1 shows a schematic of McNair’s home-built GC instrument. A complete description of the construction can be found in the thesis. A few construction details about this instrument provide lessons about gas chromatographs and columns that are still relevant today. McNair describes making and packing the columns using 6–10 ft lengths of copper tubing, which can easily be coiled, handled, and fitted into the instrument. Although stainless steel and glass columns would be more inert, McNair was thinking practically about ease-of-use. Today’s fused-silica capillary columns are designed with ease-of-use in mind as well. Other materials might be more inert and more temperature stable, but fused silica columns are easy to use.
FIGURE 1: Schematic diagram of McNair’s original laboratory-built gas chromatograph. Adapted and updated from reference (1).
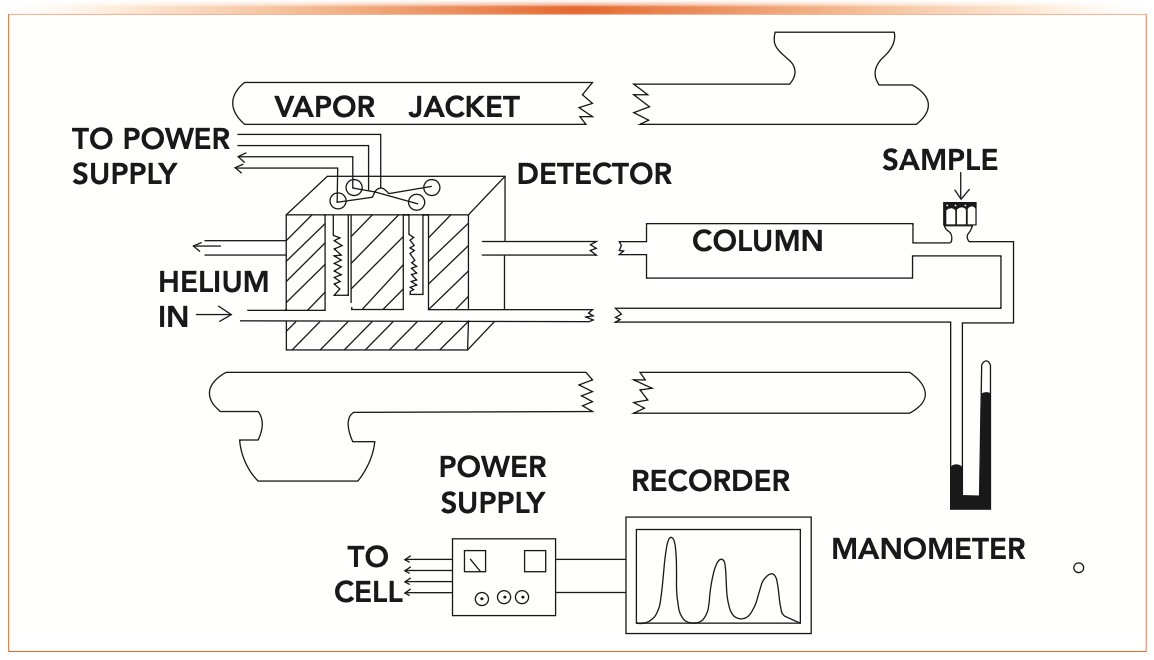
McNair’s GC instrument did not have a column oven as we have come to know them. Traditional ovens of the day at a reasonable cost would not likely have provided precise enough temperature control for systematic and reproducible studies of retention. In McNair’s gas chromatograph, the column and detector were placed in a vapor jacket that contained the vapor of a refluxing solvent at constant temperature. Using acetone, water, and anisole provided stable constant temperatures at 55 °C, 100 °C, and 153 °C, respectively. Although this arrangement provided stable temperatures, it was obviously difficult to change temperature. Temperature programming, a new concept at the time, was out of the question.
McNair recognized the need for strong analyte retention in studies of stationary phase properties, so he prepared packed columns using 30% by weight of each stationary phase with the other 70% being the solid support particles. He commented that this was a higher than usual loading of the stationary liquid phase onto the solid particles, to ensure greater retention of the analytes through having a higher mass of stationary phase in the column. Today, the equivalent thinking is to use a thicker film capillary column to increase retention.
Finally, McNair provides a summary of classical early works on chromatography, some from before the beginnings we think of today. He notes an original work describing gas–solid chromatography but not described as chromatography, as it predates Tswett’s coining of the term chromatography by several years (3,4). In 2010, McNair provided an excellent summary of the history of GC for LCGC North America (5).
McNair’s classic book, Basic Gas Chromatography, was first published in 1964 and became the standard by which all other books intended for new users to learn analytical instruments is measured (6). Figure 2 is a photograph of my own well-worn copy that I purchased upon joining his research group in the 1980s. Of course, this book was required reading in his courses and at the time it still contained much timely information. Today, the original versions with the instantly recognized classical green cover seem dated but the concise descriptions of stationary phases, detectors, and troubleshooting are still relevant. Over the decades, the original Basic Gas Chromatography sold more than 130,000 copies and was published in eight languages. The original paperback is out of print, but second-hand copies are often available through online booksellers. The most recent edition, published by John Wiley and Sons, with me and Professor James Miller as co-authors, was published in 2019.
FIGURE 2: Image of the original green cover of the book entitled Basic Gas Chromatography.

The more modern discussion of stationary phase chemistry and polarity presented in the later editions Basic Gas Chromatography demonstrate the continuing timeliness and relevance of this discussion, which began with McNair’s thesis and was re-energized with the development of ionic liquid–based stationary phases in the 2000s (7). When these highly polar stationary phases were being developed, it was quickly discovered that the chemical names of the compounds used to make them were far too complex for marketing purposes, or even for most chemists to remember. In 2011, to simplify the discussion of the polarity of these new stationary phases, Mondello seized on an idea presented in Basic Gas Chromatography that an overall stationary phase polarity value could be generated using the sum of the McReynolds constants for that phase (8). Mondello’s polarity numbers, calculated from the sum of McReynolds constants, are now widely used to express the overall polarity of stationary phases (9).
Since its start in 1965 and written in a style that does not require the reader to be a highly experienced chemist, Basic Gas Chromatography has been the “go-to” first book on GC for analysts all over the world.
McNair’s publications also provide insight into major developments in chromatography over the years. Although he is best known as a pioneer in GC, McNair’s publication list includes many important works in high performance liquid chromatography (HPLC), supercritical fluid extraction (SFE), supercritical fluid chromatography (SFC), capillary electrophoresis (CE), mass spectrometry (MS), and many more techniques. Perhaps the most important lesson from McNair’s publication list is to not just focus on a single technique, but think about solving problems. Thinking about or learning one technique is too limiting for the complex scientific problems of today.
Chromatography and Computers
In 1972, McNair hosted the first International Symposium on Computer Chromatography and Associated Techniques in Mainz, Germany. His editorial introducing the symposium, which was captured in a special issue of Chromatographia, included several comments about data systems and chromatographic data analysis that remain relevant today (10). McNair asked the following two questions: “What are we doing with computers?” and “What should we be doing with computers?” The symposium volume provides an excellent set of answers to the first question, which included developing interfaces for single and multiple instruments, acquiring the data, and processing the data. McNair commented that the introduction of computers to chromatography caused chemists to complain about the large amounts of data generated each morning. This complaint rings true today in the fast GC and comprehensive two-dimensional gas chromatography (GCxGC) communities, which often deal with large data sets. A few of the article titles from this 1972 symposium, including Kaiser’s “PPB-Analysis Computerized?” and Battista’s “Computerized Blood Alcohol Analysis” demonstrate the intense interest in quantitative analysis. Several other articles on data analysis and evaluation include diagrams showing integration schemes for overlapping and asymmetrical peaks that look very similar to those used to describe these processes in today’s modern data systems.
McNair emphasized the need to use computers for qualitative analysis, mentioning the early work of Schomburg and Ziegler on the use of Kovats retention indexes to identify analytes. These early ideas led to the development of retention index libraries that are still in common use today. I was fortunate to work with McNair and Sadtler Research Laboratories in the 1980s on the development of their retention index library into a “poor man’s gas chromatography–mass spectrometry (GC–MS)” concept, whereby retention indexes measured on two significantly different stationary phases could be compared to libraries to generate an accurate qualitative analysis. With the rise of benchtop GC–MS and GCxGC–MS instruments, tools for qualitative analysis have been included in many of today’s data systems. McNair’s comments about qualitative analysis and large data sets still resonate in the GCxGC community, where both are the norm, and the chromatograms themselves are increasingly complex.
LC versus GC
In 1974, McNair posed a question that is still commonly asked today in some form: “How soon will liquid chromatography replace gas chromatography?” (11). He then comments that asking the question at all may demonstrate a general lack of understanding about the two techniques. Difficulties in fundamental understanding exist today. In a comprehensive edited volume on GC published this year, Poole devotes a short section and commentary to this challenge (12). Interestingly, having published exclusively on GC until then, McNair built an extensive publication record on HPLC and other techniques, including GC. When I was in his group, there were two active laboratories in the group: the “LC group” and the “GC group.” The LC and GC laboratories were even in separate buildings for a time. McNair kept us all working together: LC group members worked with and challenged GC group members and vice versa He made sure we were all cross-trained and that we all fundamentally understood separation science, not just individual techniques, and problem solving. The answer to the question of LC replacing GC was never fully decided, and McNair’s response is as true today as it was back in 1974. McNair’s concise two-page comparison of LC and GC, including thoughts on the separation principle, sample types, minimum detectable quantities, analysis time, theoretical plates, preparative capability, and price range, is as useful today as it was back then. Viewing LC and GC as complementary highlights an important principle of analytical chemistry—that the problem should be considered before the technique, and the technique should be designed to solve the problem, not the other way around. Viewing LC, GC, and other analytical methods as competitive to one another only limits the work and experience to a single technique, which is an elementary mistake new analytical scientists can make.
In 1984, Bowermaster and McNair demonstrated the fundamental complementary nature of LC and GC, describing temperature programming in HPLC (13). In both GC and LC, partitioning between the mobile and stationary phases is an equilibrium- controlled process so both techniques are subject to similar phase thermodynamic principles that govern retention. As a simple analogy, mobile-phase strength in LC is like temperature in GC. However, in LC, temperature can also be used as an additional variable to control retention. Temperature-programmed versus isothermal LC runs showed very similar behavior to temperature-programmed runs in GC, with the temperature-programmed runs showing roughly equal spacing of the peaks versus the exponential spacing seen in isothermal runs. Although temperature-programmed HPLC has not caught on very much, precise temperature control, obviously necessary in GC, which Bowermaster and McNair also discussed in detail, has been incorporated into nearly all of today’s commercial HPLC systems.
These brief vignettes provide a small taste of Professor Harold McNair’s massive contributions to separation science. His publication record alone does not capture the whole story of a career and life devoted not only to the science but to people all over the world. There are few separation scientists of my own and previous generations that did not meet or interact with McNair at a conference, a short course, or in the laboratory. He was quick to invite visitors from all over the world to visit his laboratory in Blacksburg, Virginia to share knowledge, experience, and good times. He was a world traveler who routinely visited the laboratories of colleagues all over the globe. We had a globe of the earth in our laboratory with a small arrow attached, pointing to wherever in the world “Doc” was, often not Blacksburg. Whether through publication, working in the laboratory together with other scientists, or from conversations, Harold McNair freely shared his knowledge and experience. His work and spirit influenced separation science far beyond GC and he is easily considered among the most influential analytical scientists of the past 60 years.
Thank you, Harold.
References
(1) H.M. McNair, “Efficiency of Solvents in Gas Chromatography” PhD Dissertation, Purdue University, 1959. Retrieved from https://www.proquest.com/dissertations-theses/efficiency-solvents-gas-chromatography/docview/301871733/se-2?accountid=13793
(2) J.V. Hinshaw, LCGC N. Am. 20(11), 1034–1040 (2002).
(3) W. Ramsey, Proc. Royal Soc. A. 76A, 111–114 (1905).
(4) M. Tswett, Ber. Deut. Bot. Ges. 3316, 384 (1906).
(5) H.M. McNair, LCGC N. Am. 28, 138–144 (2010).
(6) H.M. McNair and E.J. Bonelli, Basic Gas Chromatography (Varian, Palo Alto, 1964).
(7) H. Nan, TrAC Trend Anal. Chem. 105, 367–379 (2018).
(8) C. Ragonese, D. Sciarrone, P.Q. Tranchida, P. Dugo, G. Dugo, and L. Mondello, Anal. Chem. 83, 7947–7954 (2011).
(9) “Selecting a GC Column by a Specific Stationary Phase” Millipore Sigma, 2021. https://www.sigmaaldrich.com/US/en/technical-documents/protocol/analytical-chemistry/gas-chromatography/specific-stationary-phase? (accessed August 2021).
(10) H.M. McNair, Chromatographia 5(2–3), 61 (1972).
(11) H.M. McNair, Chromatographia 7, 161 (1974).
(12) C.F. Poole (ed), Gas Chromatography, 2nd Edition (Elsevier, Amsterdam, The Netherlands), pp. 15–16.
(13) J. Bowermaster and H.M. McNair, J. Chromatogr. Sci. 22(4), 165–170 (1984).
About the Author
Nicholas H. Snow is the Founding Endowed Professor in the Department of Chemistry and Biochemistry at Seton Hall University, and an Adjunct Professor of Medical Science. During his 30 years as a chromatographer, he has published more than 70 refereed articles and book chapters and has given more than 200 presentations and short courses. He is interested in the fundamentals and applications of separation science, especially gas chromatography, sampling, and sample preparation for chemical analysis. His research group is very active, with ongoing projects using GC, GC–MS, two-dimensional GC, and extraction methods including headspace, liquid–liquid extraction, and solid-phase microextraction. Direct correspondence to: LCGCedit@mmhgroup.com.


Detecting Hyper-Fast Chromatographic Peaks Using Ion Mobility Spectrometry
May 6th 2025Ion mobility spectrometers can detect trace compounds quickly, though they can face various issues with detecting certain peaks. University of Hannover scientists created a new system for resolving hyper-fast gas chromatography (GC) peaks.
Altering Capillary Gas Chromatography Systems Using Silicon Pneumatic Microvalves
May 5th 2025Many multi-column gas chromatography systems use two-position multi-port switching valves, which can suffer from delays in valve switching. Shimadzu researchers aimed to create a new sampling and switching module for these systems.
New Study Reviews Chromatography Methods for Flavonoid Analysis
April 21st 2025Flavonoids are widely used metabolites that carry out various functions in different industries, such as food and cosmetics. Detecting, separating, and quantifying them in fruit species can be a complicated process.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)












