Origins of Sample Preparation Technologies
In this column, and elsewhere throughout the research community devoted to chromatographic sample preparation, the importance of the knowledge of the fundamental principles underlying sample preparation is emphasized. What is the role of solubility, diffusion, surface tension, and other parameters on the efficacy of extraction, selectivity, and so on? Of course, the answer is partly based on insight into the physicochemical principles upon which they are based, and partly based on serendipity. This month, we take a look at some of the more popular sample preparation methods, and present a discussion of their initial conception and development, to shed light on their current roles in our analytical “toolbox.”
Two years ago, we presented a retrospective look at the classical Soxhlet extraction, including the origins, operation, standard methods, and derivations (1). One can learn about the principles of modern techniques for extracting solid samples, such as via supercritical fluid extraction (SFE), pressurized liquid extraction (PLE), and microwave-assisted extraction (MAE), from a working knowledge of Soxhlet extraction, according to our premise. Now, let’s take it to the next step and investigate the origins of more modern treatments of extraction. To do this, we look to the chemical literature, but more importantly, some of the folklore is presented based on my conversations or subsequent communications with key players in the field of sample preparation. Last year, my colleague Brian Logue described his thought process in combining freeze concentration with stir-bar sorptive extraction (SBSE) to form a novel approach called ice concentration linked with extractive stirrer (ICECLES) (2). This is a great example of combining natural curiosity and an understanding of fundamental principles to develop modern approaches to analytical problem solving. Stories about the development of modern analytical extractions follow.
Solid-Phase Extraction
Perhaps the place to start is the beginning...of chromatography. Of course, A.J.P. Martin and R.L.M. Synge are foremost among the pioneers of analytical separations. In 1941, they separated amino acids via partition between aqueous and organic phases by designing a “mixer-settler” extractor (3). They abandoned this approach when they encountered mechanical difficulties with their apparatus, and turned their attention toward the development of liquid–liquid (partition) chromatography (LC). However, L.C. Craig picked up on this work in refining his countercurrent extractor.
As a side note, when I was in graduate school, Milton Lee always showed a Craig countercurrent apparatus during his separations course. Each time, one or more of the glass tubes broke off. I feel sorry for the more recent students that probably never observed a somewhat intact device.
We start this discussion with the advent of liquid chromatography (LC), because in 1977, James Waters, founder of the LC company that bears his name, challenged company scientist Patrick McDonald to “find new, faster, more convenient ways to do traditional sample preparation operations (4).” McDonald proposed to “solve a real analytical problem using, whenever possible, our LC technology.” The team at Waters used their silica-based adsorbent technology, along with triaxial bed compression and individual cartridge packaging to create the SEP-PAK product. Within a few years, countless competitors began appearing and the J.T. Baker Company rearranged the SEP (which stood for “Sample Enrichment and Purification”) acronym into SPE, or the more generic solid-phase extraction. This application of LC technology to analytical sample preparation was forward thinking to the extent that many of us are still asking the questions posed by Waters in their original marketing literature; see Figure 1 (4).
FIGURE 1: Questions posed by analysts in evaluating sample preparation technologies, as posed by Waters Corporation during the introduction of SEP-PAK Cartridges.

QuEChERS
One of the most recent, and more intriguing, techniques in the sample preparation arsenal is that known as QuEChERS or quick, easy, cheap, effective, rugged, and safe extraction, developed in the 2000s. This approach has been adapted for use with the extraction of almost any analyte from almost any matrix, or so it seems. Steve Lehotay in the U.S. Department of Agriculture’s Agricultural Research Service laboratory in suburban Philadelphia and his colleague Michelangelo Asastassiades at the EU Reference Laboratory for Pesticides set out to develop what Lehotay described in an email message as, “the most efficient method for multiclass, multiresidue analysis of pesticides in foods.” (5)
Previously, both researchers had explored SFE and taxed it to its limits. Steve describes their collaboration as something like, “the Holy Grail of regulatory and industry laboratories for decades.” He explained further:
Increasing concerns about excessive glassware, use of chlorinated solvents, high labor needs, and low sample throughput were putting more pressure on laboratories to adopt more efficient practices. More sensitive, smaller, and less expensive gas chromatography–mass spectrometry (GC–MS) and liquid chromatography–tandem mass spectrometry (LC–MS/MS) instruments were also being introduced at about that time, which permitted an even broader scope of analysis while still maintaining high selectivity in detection. This meant that sample preparation needed to recover a wide range of polar and nonpolar pesticides, but cleanup was still needed to avoid instrument contamination. (5)
Ultimately, it became a brute force effort. They were aware of magnesium sulfate as both a drying agent and an extraction aid, so that was a starting point. They isolated and studied solvent type, ratio of sample size to solvent volume, sample and buffer pH, salting-out salts and amounts, and extraction time and temperature. They determined recoveries depending on analyte polarity relative to the amounts of water and solvent used, along with measuring co-extractives. After tailoring the extraction for a broad range of pesticides and food samples, their attention turned to the dispersive-SPE step, addressing the perceived disadvantages of matrix-solid phase dispersion, the cost of cartridges, and related factors. Since that time, several vendors have modified the salts or parameters to expand the scope of the technique, and Lehotay has refined the approach to a new QuEChERSER which will be the subject of an upcoming "Sample Prep Perspectives" column.
In the food industry, it is customary to name extraction techniques after those who developed them, such as the Luke or Randall methods. However, the inventors apparently felt that the “Lehotay and Asastassiades Method” didn’t roll off the tongue with the same impact as listing the key attributes of the technique!
Solid-Phase Microextraction
Continuing along the theme of extraction techniques based on chromatographic sorbents is solid-phase microextraction (SPME). Despite its name, SPME is not a miniaturized version of SPE. It is described as an open bed, diffusion rate-controlled technique (6). While numerous versions of the approach continue to appear, our discussion will focus on the original, traditional variant of SPME, and this is perhaps a case where sometimes a researcher can be too advanced.
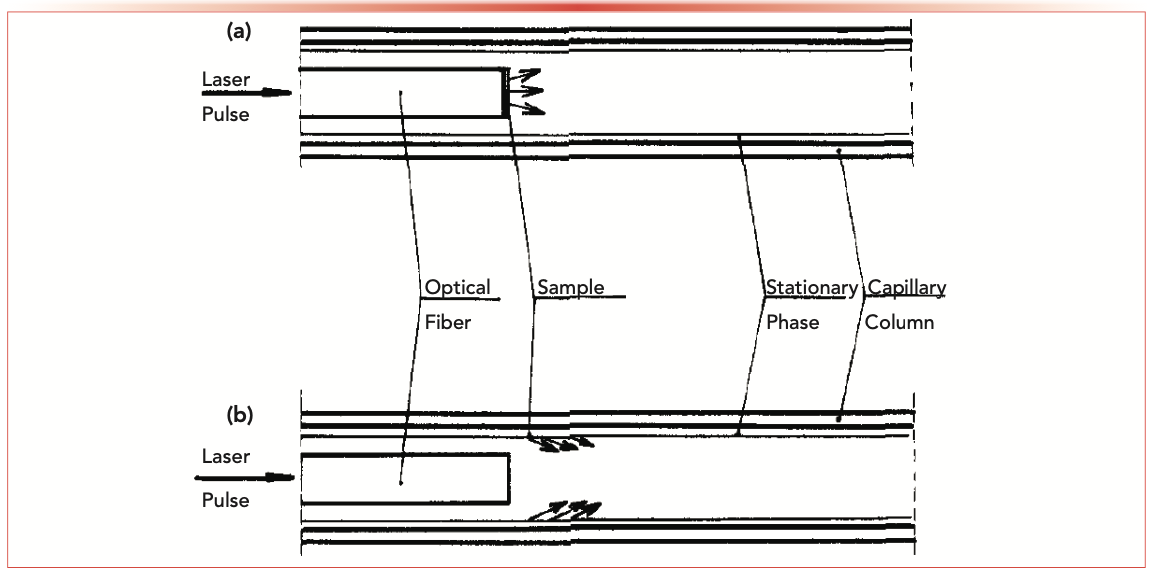
The story of SPME begins in 1987, when Pawliszyn and Liu described sample introduction in capillary gas chromatography (GC) using laser desorption of the sample from the end of an optical fiber, as seen in Figure 2 (7). This provided inspiration to Pawliszyn, who realized that the fiber, clad with a stationary phase like polydimethylsiloxane (PDMS), could also extract organic compounds. The fiber optic replaced the wire used in microsyringes.
FIGURE 2: Depiction of laser desorption from the end of an optical fiber for sample introduction into capillary GC. Reproduced with permission from reference (7).
Since then, SPME continues to develop, with myriad configurations providing unique advantages. For example, thin-film SPME, the SPE Arrow, and SBSE all provide enhanced sample loading and sensitivity compared with the original device. One distinctive format is the coated-blade spray, also developed in Pawliszyn’s laboratory. Coated-blade spray builds from the substrate-spray ionization approaches to MS developed by John Fenn at Yale University, and later by Virginia Commonwealth University, Jentaie Shiea in Taiwan, and Graham Cooks at Purdue University. Coated-blade spray marries SPME and substrate-spray ionization for direct-to-MS analysis. A flattened, sword-shaped stainless steel blade is coated with stationary phase, much like SPME but with a broader array of phases. Then, after the sample is applied, an electrospray solution is added, and the device receives a high potential difference across the uncoated portion of the blade, resulting in electrospray ionization in the mass spectrometer.
Stir-Bar Sorptive Extraction
SBSE represents an interesting example of applying your expertise to a seemingly unrelated situation, at least partially according to the folklore. We remember overhearing a conversation involving Pat Sandra from Belgium’s Research Institute of Chromatography, where he claimed that his inspiration was reading one of his colleague’s papers where, in performing a mass balance for an extraction, the author looked at sample losses due to volatilization, adsorption to the walls of the glassware and stir bar, and other reasons. Sandra thought he could apply a stationary phase to the stir bar and intentionally adsorb the analyte onto the device for subsequent desorption. There is more to this story, again demonstrating knowledge of fundamental principles reapplied in a novel manner.
David, Ochiai, and Sandra note that, in the early days of SPME, a controversy existed regarding the nature of the sorption phenomenon observed (8). Apolar solutes could potentially adsorb onto the Teflon-coated stir bar used for sample agitation, as well as onto the PDMS SPME coating. This led to the development, and subsequent commercialization, of PDMS-coated stir-bars for extraction, the advent of SBSE. In the two decades since Sandra’s keen insight, approximately 1000 reports of SBSE are in the literature.
Pressurized Liquid Extraction
The 1990s are considered the heyday of the development of modern instrumental approaches to sample preparation, including SFE and MAE. In the early part of that decade, Dionex/Lee Scientific introduced the Model 703 supercritical fluid extractor. Within a very short period, their competitors responded with their SFE offerings and with the more advanced developments, shortcomings in the earlier equipment rose to the forefront. In response, the Dionex Salt Lake City Technical Center, home to the SFE development, convened a customer focus group to “fix the SFE.” Led by Bruce Richter and Brian Jones, the conversation led to questions like “If nearly all SFE requires the use of an organic co-solvent, what is the role of the carbon dioxide?” From here, the concept evolved into the concept of an “analytical pressure cooker”—that is, the application of pressure to an extracting solvent with the intent of increasing its boiling point to create enhanced extracting conditions. Both kinetic and thermodynamic factors become favored at temperatures of 100–150 °C. The resulting technique became Accelerated Solvent Extraction (ASE), a trade name more generally referred to as PLE. During the development of SFE instrumentation in the 1990s, the U.S. Environmental Protection Agency’s Office of Solid Waste issued a memo to equipment manufacturers delineating the requirements to gain approval for an official EPA method utilizing SFE. Dionex followed this guidance during their development of the ASE technique, and they received early market acceptance. As a result of the introduction of ASE, not only did other vendors follow suit in creating competitive instrumentation, but the overhype of SFE declined. Applications of SFE became less risky and increasingly oriented toward those applications, like foods, flavors, or polymers, where supercritical fluids truly had advantages.
Vaping as Sample Preparation
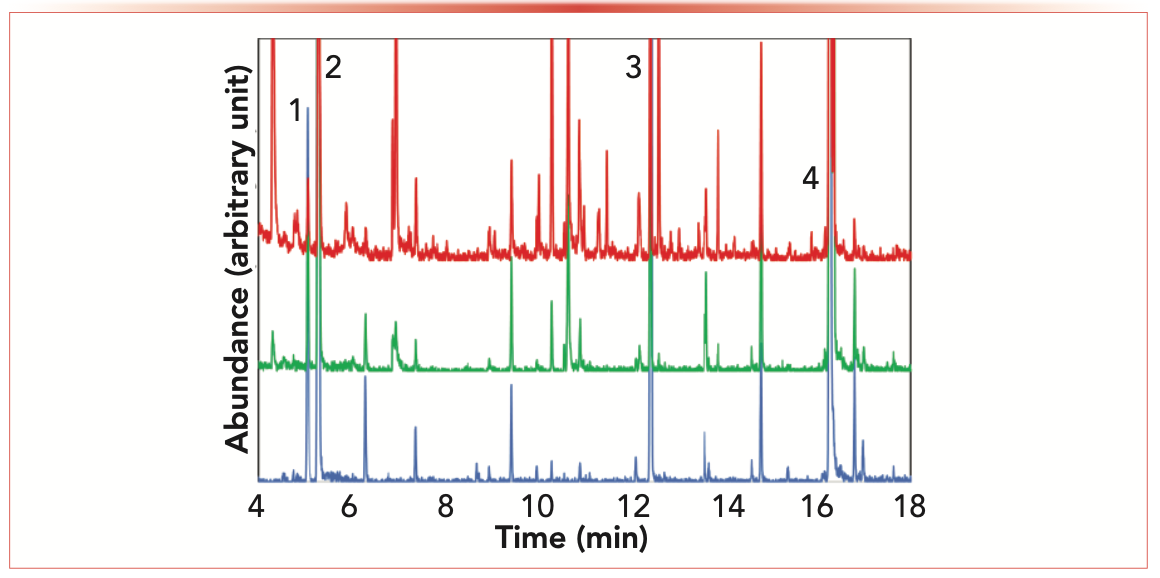
Finally, we introduce a novel sample preparation approach based on knowledge of extraction fundamentals and creative reapplication of casual observations. While I know little about the phenomenon of vaping, I realized that this approach is fundamentally sample preparation. The vaping device, via application of elevated temperature, vaporized sample components for delivery by inhalation. We obtained a dry herb vaporizer and, as the final part of his dissertation (8), one of our graduate students, Ahsan Ahmed, evaluated its use in analytical extractions. The particular device we used allowed controllable temperatures up to 240 °C. What was intriguing was that, not only could we employ temperatures similar to conventional headspace techniques and thermal desorption, but also at often unexplored temperatures intermediate between these techniques and pyrolysis. Ahsan used the vaping pen with little modification and SPME to collect the emitted vapors. Due to the volume of the vaping chamber, improved sensitivity resulted. Figure 3 shows the GC–MS results for the extraction, or vaping, of horseradish root as a function of temperatures from 150 to 240 °C. While further investigation is warranted and improvements to the device may be necessary, it shows promise for use in analytical laboratories, perhaps even for applications done “in the field.” Our initial paper describing this approach, our application to food and environmental samples, and comparison to headspace sampling is in preparation, with the intent of journal submission this fall.
FIGURE 3: GC–MS results demonstrate the use of a dry herb vaporizer for the extraction of horseradish root at 150 °C (blue), 200 °C (green), and 240 °C (red).
Conclusions
We’ve presented the told and untold stories surrounding the development of several modern extraction techniques. These examples confirm that the invention process requires understanding of the fundamental principles in a given field, often reapplied in a unique setting. While some of these reapplications may seem intuitive in retrospect, what is obvious to one investigator may not be so to others. Of course, serendipity also plays a role in the creative process. Perhaps, sometime down the road, readers of this column may be inspired to develop new approaches by employing their knowledge and experience.
References
(1) D.E. Raynie, LCGC North Am. 37, 510–513 (2019).
(2) B.A. Logue, C. Skaggs, and A.H. Alluhayb, LCGC North Am. 38, 16–22 (2020).
(3) B.S. Solomon, in A History of Analytical Chemistry, H.A. Laitinen and G.W. Ewing, eds. (American Chemical Society, Washington, DC, 1977), pp. 292–326.
(4) P.D. McDonald, “James Waters and his Liquid Chromatography People: A Personal Perspective,” http://www.waters.com/webassets/cms/library/docs/wa62008.pdf.
(5) S. Lehotay, personal communication.
(6) N. Reyes-Garces, E. Gionfriddo, G.A. Gomez-Rios, M.N. Alam, E. Boyact, B. Bojko, V. Singh, J. Grandy, and J. Pawliszyn, Anal. Chem. 90, 302–360 (2018).
(7) J. Pawliszyn and S. Liu, Anal. Chem. 59, 1475–1478 (1987).
(8) F. David, N. Ochiai, and P. Sandra, TrAC Trends Anal. Chem. 112, 102–111 (2019).
(9) A. Ahmed, Ph.D. dissertation, South Dakota State University, Brookings, South Dakota (2021).
About the Column Editor
Douglas E. Raynie
“Sample Prep Perspectives” editor Douglas E. Raynie is a Department Head and Associate Professor at South Dakota State University. His research interests include green chemistry, alternative solvents, sample preparation, high-resolution chromatography, and bioprocessing in supercritical fluids. He earned his PhD in 1990 at Brigham Young University under the direction of Milton L. Lee. Raynie is a member of LCGC’s editorial advisory board. Direct correspondence about this column via e-mail to LCGCedit@mjhlifesciences.com


Thermodynamic Insights into Organic Solvent Extraction for Chemical Analysis of Medical Devices
April 16th 2025A new study, published by a researcher from Chemical Characterization Solutions in Minnesota, explored a new approach for sample preparation for the chemical characterization of medical devices.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)






