Evaluating Two Dioscin-Based Silica Stationary Phases and their Application to Achiral and Chiral Separations
In a previous study, polyphyllin III, an important natural dioscin ingredient, was bonded to silica particles using the “one-pot method” for preparing a stationary phase, called SP-D. SP-D was further modified to produce two stationary phases, namely the phenyl isocyanate-dioscin (Phe-D) and 3,5-dimethyl phenyl isocyanate-dioscin bonded silica stationary phase (DMP-D), respectively. The Phe-D and DMP-D were evaluated by achiral and chiral analytes. The high performance liquid chromatography (HPLC) method was optimized and applied to the analysis of the main active ingredient, polyphyllins, that were contained in gongxuening capsules, a traditional Chinese medicine (TCM) widely used to treat gynecological diseases. The two synthesized stationary phases were also applied to the separation of the amino acid (AA) enantiomers. For this study, 10 α-AAs (lysine, leucine, cysteine, arginine, isoleucine, threonine, serine, valine, alanine, and histidine) were selected and studied for chiral separation using Phe-D and DMP-D stationary phases. In this study, 10 observed AA enantiomers were separated to different degrees on at least one stationary phase whereas four AAs were enantioseparated on both Phe-D and DMP-D. These results indicated that the two synthesized stationary phases have potential applicability in quality control (QC) of TCM and chiral separation as well as offering a new application choice for analyses of natural products.
As the pharmaceutical and natural medicine industries continue to evolve, new separation issues posed by various samples need to be resolved. As a result, the demand for more reversed-phase high performance liquid chromatography (HPLC) stationary phases is becoming significant. The ongoing research in designing, synthesizing, and testing the performance of stationary phases, is therefore crucial (1,2).
It is possible to prepare a stationary phase that possesses both support and active functions by bonding the active ingredients to the surface of the silica gel base material. Some stationary phases with ligands extracted from medicinal plants such as emodin, curcumin, saponin, magnolol, allicin, quercetin, and isatin have been prepared (3–6). This type of stationary phase can provide not only the functions of hydrophobic interaction, but also hydrogen-bonding interaction, π-π, and n-π conjugation. They are quick and highly selective for structurally similar compounds (7–9). Modified or derivative natural products can be bonded to the surface of the silica gel to prepare HPLC chiral stationary phases (CSP). These CSPs, based on products such as cyclodextrins, polysaccharides, and macrocyclic glycopeptide antibiotics and proteins, were proven to be highly efficient (10–15). The interactions, hydrogen-bonding, π-π stacking, inclusion interaction, and ion exchange, between the solute and CSP played a key role in chiral resolution (16,17).
Dioscin, one active compound in Dioscoreae rhizoma, and several Discordance plants, including Dioscorea zingiberensis C.H. Wright (D. zingiberensis) and Dioscorea nipponica Makino (DNM), possess anti-inflammation, immunoregulation, hypolipidemic, antiviral, antifungal, and antiallergic effects (18). Dioscin is also used as an important ingredient for the synthesis of various steroid hormone drugs.
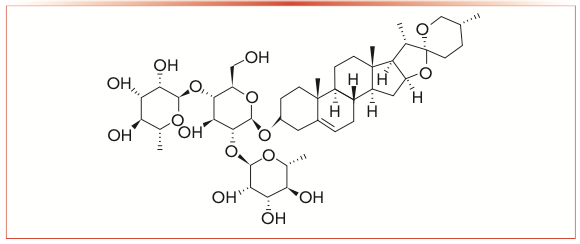
Figure 1 shows the structural formula of dioscin. As the figure shows, dioscin contains active hydroxyls that provide favorable conditions for binding events. The multiple hydroxyls and double bonds can participate in hydrogen-bonding interaction, dipole–dipole interaction, and π-π interactions, and the structure is large enough to shield the influence of the acidic Si-OH residue on the stationary phase and improve the separation process. The plane and rigid structure of dioscin can also improve the stereoselectivity by providing a larger contact surface for the solute molecules. In this case, we bonded dioscin to silica gel to obtain a dioscin-bonded stationary phase, called SP-D. Then, two derived stationary phases were produced, namely phenyl isocyanate-dioscin bonded silica stationary phase (Phe-D) and 3,5-dimethyl phenyl isocyanate-dioscin bonded silica stationary phase (DMP-D), respectively. In a previous study, these two derived stationary phases were evaluated, and it was determined that they had typical reversed-phase (RP) chromatographic performance, which makes both similar to the performance of octadecylsilyl (ODS) columns (19).
FIGURE 1: Structural formula of dioscin.
Quality control (QC), including qualitative and quantitative analysis, is a major bottleneck for traditional Chinese medicines (TCM) with their increased use worldwide (20). There are many ingredients, including active ingredients, auxiliary components, and inactive ingredients in TCM. Because of the sheer quantity of ingredients in TCM, it is too difficult to identify and characterize all of them. Therefore, a characteristic HPLC chromatogram is always chosen to compare and evaluate TCM (21,22). For quantitative analysis, a single component cannot represent the full efficacy of any TCM. Analysis of multiple active components, through quality markers (Q-markers) detection is therefore necessary (23,24). As a result, the combination of a characteristic chromatogram and quality markers detection is a powerful way to ensure quality of TCMs.
Chirality caused by molecular asymmetry has special effects on physical, chemical, biological, and pharmacological properties appearing at a molecular level (25). Amino acids (AA), the simplest chiral molecule, have at least one stereogenic center (with the exception of glycine). The important analytical task of the chiral separation of an amino acid is achieved mainly by chromatographic methods, especially HPLC, including direct and indirect separation (26).
In a previous study, we prepared two natural active ingredient dioscin-based stationary phases, Phe-D, and DMP- D, respectively. The bonding method, the “one-pot method,” used to produce the stationary phases directly without tedious intermediate steps, was proven to be simple and convenient. The Phe-D and DMP-D stationary phases were characterized by elemental analysis, scanning electron microscopy, thermogravimetric analysis, and infrared (IR) spectroscopy. These new stationary phases were evaluated by benzene and its homologs, polycyclic aromatic hydrocarbons, and other solutes as probes. Through these evaluations, Phe-D and DMP-D were verified to have typical RP chromatographic performance, similar to that of ODS columns, and the separation principle was shown to be related to the hydrophobic effect. In addition, these stationary phases were confirmed to have the ability to provide hydrogen bonding and π-π conjugation sites for solutes (19).
To evaluate and compare these two stationary phases, we investigated their application to achiral and chiral separation. In this study, we used HPLC characteristic chromatograms of 10 batches of gongxuening capsules and determined the content of six main polyphyllins in 10 batches of gongxuening capsules. Then, the application of Phe-D and DMP-D to chiral separation of 10 amino acids was studied and evaluated.
Experimental
Reagents and Chemicals
Polyphyllin I and II (Guizhou Dida Biochemistry Co.), polyphyllin III (Nanjin Biochemistry Co.), polyphyllin V (Chengdu Herbpurify Co., Ltd.), polyphyllin VI and VII (Sichuan Weikeqi Biotech Co., Ltd.), and gongxuening capsules (Yunnan Baiyao Group Co., Ltd.) were used. The AA enantiomers lysine, leucine, cysteine, and arginine came from Shanghai Lanji Technologies Co., Ltd. The AA enantiomers isoleucine and threonine came from the Chinese Academy of Sciences’ Institute of Biochemistry. For the remaining AA enantiomers, serine came from the Shanghai Chemical Reagents Procurement Station, valine came from Feinbiochemical Heidelberg, alanine from the Shanghai Third Reagent Factory, and histidine from the Shanghai Renfa Comprehensive Utilization Factory.
All the other reagents were analytically pure and procured through commercial channels.
Experimental Instrument and Chromatographic Conditions
The Agilent 1260 HPLC system, which included the G1322A Vento, a G1312C delivery pump, a G1329B standard automatic sampler, a G1316A column oven, a G1315D detector, and the Agilent 1200 LC chromatographic work station, was used as the instrumentation for this study. The separation was performed nate-dioscin bonded silica gel stationary phase, 4.6 × 250 mm; and DMP-D: 3,5-dimethyl phenyl isocyanate-dioscin bonded silica gel stationary phase, 4.6 × 250 mm). The mobile phase consisted of acetonitrile and 0.01% phosphoric acid for QC, and for the chiral separation, methanol and a 0.04 mol/L buffer solution were used. The flow rate was 0.4 mL/min. The injection volume was 10 μL. The column oven was maintained at 35 °C and the ultraviolet (UV) detector was set at 203 nm for quality control. The column oven was maintained at 25 °C, and the UV detector was set at 210 nm for chiral separation. Column efficiency was determined with biphenyl, the theoretical plate number of Phe-D is 4776 block/m, and the theoretical plate number of DMP-D is 5028 block/m.
Preparation of Reference and Sample Solutions
Preparation of Reference Solutions
The appropriate amount of Polyphyllin I, II, III, V, VI, and VII were dissolved in methanol to prepare the reference solutions of various concentrations (0.375 mg/mL, 0.375 mg/mL, 0.400 mg/mL, 0.300 mg/mL, 0.450 mg/mL, and 0.450 mg/mL, respectively).
Preparation of Sample Solutions
The content of 20 capsules from 10 different batches of gongxuening capsule was weighed and porphyrized. Next, 1 g of the substance was added into a conical flask with cover along with 25 mL of methanol. The flask was then sealed and weighed. This solution in the flask was treated ultrasonication for 40 min and then cooled to room temperature. Afterward, methanol was added to the solution to compensate any weight loss. The solution was shaken well and filtered with a 0.22 μm microfiltration membrane to remove particulates and the subsequent filtrate was kept as the sample solution.
Preparation of Sample and Buffer Solution for Chiral Separation
Racemic lysine, leucine, cysteine, arginine, threonine, serine, valine, alanine, isoleucine, and histidine were separately dissolved in water, and the solutions were adjusted to the appropriate concentration and then filtered with a 0.22-μm microfiltration membrane. The appropriate amount of ammonium acetate was dissolved in water and titrated with glacial acetic acid at the desired pH to make solutions of 0.04 mol/L.
Determining Linearity, Precision, Recovery, and Stability
A series of mixed reference solutions with polyphyllin I, II, III, V, VI, and VII at five levels (3, 6, 9, 12, and 15 μL) were analyzed. The standard curve was drawn with the concentration (x) of reference solution as the abscissa and the area of chromatographic peak (y) as the ordinate. Linearity was evaluated using a linear calibration curve and by calculating the coefficient of determination. A mixed reference solution with polyphyllin I, II, III, V, VI, and VII with the volume of 10 μL was analyzed six times. Each peak area was recorded for the real standard deviation (RSD) calculation.
To determine the recovery rate, the reference substances (polyphyllin I, II, III, V, VI, and VII) were added to the same sample of gongxuening capsule one by one. Then, a 1.0 g spiked sample of gongxuening was used in conjunction with methanol as previously described in the “Experimental Instrument and Chromatographic Conditions” section. The amount of each reference substance in the sample was calculated by the linearity equation.
Six gongxuening capsule samples from the same batch were prepared with methanol. These solutions were injected into the HPLC column and analyzed. Each common peak area was recorded to analyze the consistency of the relative retention time and relative peak area. A sample solution was placed at room temperature and the sample solution was injected into the HPLC system after 0 h, 4 h, 8 h, 12 h, and 16 h, respectively.
Results and Discussion
The Application of Phe-D and DMP-D to Quality Control of the Gongxuening Capsules
A gongxuening capsule is comprised of the ethanol extract of the traditional Chinese herbal medicine Paris polyphylla var. yunnanensis (Franch.) Hand.-Mazz (27). It is widely used to treat diseases such as menometrorrhagia, metrorrhagia, uterine hemorrhages caused by insufficient contraction after delivery or abortion, pain in lateral lower abdomen or lower back, and morbid leukorrhea caused by chronic pelvic inflammatory diseases. It is confirmed that the main active ingredients of the gongxuening capsules are polyphyllins, such as polyphyllin I, II, and VI. The QC procedure for gongxuening capsules in the Chinese Pharmacopoeia is simple, which selects the polyphyllins V and VI as the identification references and the polyphyllin VI as the assay marker.
For HPLC method optimization, different mobile phases, including methanol–water, ethanol–water, acetonitrile–water, methanol–acetonitrile–water, ethanol–acetonitrile–water, acetonitrile–aqueous phosphoric acid, and acetonitrile–aqueous acetic acid were studied. The terminal absorption of methanol caused the baseline to drift, and excessive viscosity of ethanol increased the back-pressure of the columns, so acetonitrile was selected as the organic solvent. The combination of acetonitrile and 0.01% aqueous phosphoric acid (v/v) proved to be an optimal mobile phase with the gradient elution conditions. An HPLC method was applied by gradient elution using a) acetonitrile and b) 0.01% phosphoric acid as the mobile phase for QC. For Phe-D, results were taken at the following parameters: 0 min (40% A); 15 min (40% A); 40 min (55% A); 60 min (90% A), and at 75 min (90% A). For DMP-D, results were recorded at the following parameters: 0 min (40% A); 10 min (40% A); 35 min (60% A); 60 min (100% A); and at 75 min (100% A).
The proposed method for chromatographic analysis was validated in terms of precision, repeatability, and stability. Polyphyllin I was chosen as the internal reference peak. On both stationary phases, the precision was assessed by analyzing six replicate samples and the RSD of relative retention times (RRT) and relative peak areas (RPA), were below 0.75 and 2.34%, respectively. The repeatability test results showed that the RSD of RRTs was below 0.75%, and the RSD of RPAs was below 2.83%. Within 24 h, the RSD of RRTs were below 0.82%, and the RSD of RPAs was below 2.69%. These results showed that proposed methods for chromatographic analysis were reliable, consistent, and stable.
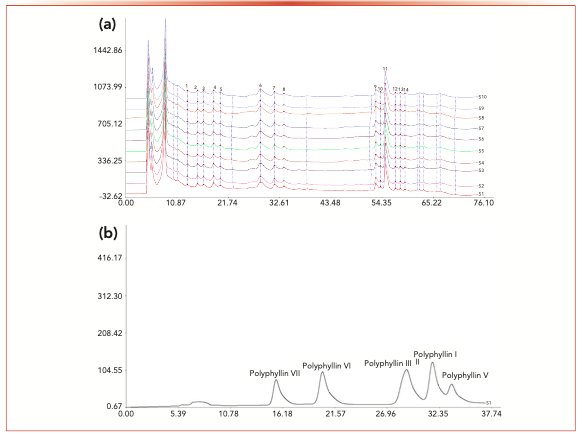
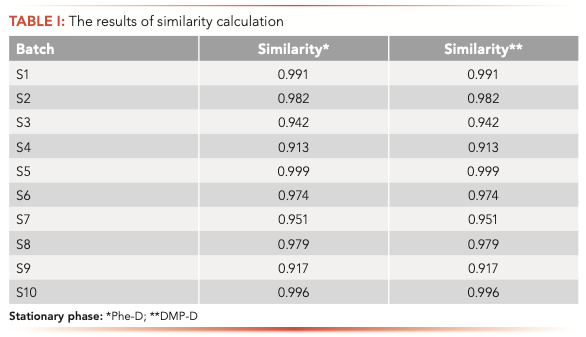
Similarity is one of the most commonly used parameters for evaluating what is termed characteristic chromatograms for TCM. Usually, similarity data for characteristic chromatograms is used to evaluate whether the quality of TCM is controllable and stable (17,18). Characteristic chromatograms obtained from 10 batches of gongxuening capsules with Phe-D are shown in Figure 2 and the similarity values, calculated by “Similarity Evaluation System for characteristic chromatograms of Traditional Chinese Medicine (version 2004A),” are reported in Table I. Each peak relative to all sample chromatograms was called the “common peak,” and 14 common peaks were observed in all 10 batches among the total 36 peaks. The peak with the retention time of 31.592 min, which was identified as polyphyllin I, was one of the most important active constituents of the gongxuening capsules and was chosen as the internal reference peak to calculate the RRT and the RPA of the other peaks. The RSDs of RRT and RPA of other common peaks with respect to Polyphyllin I from all samples were in the ranges of 0.21–0.70% and 1.84–41.15% respectively, which indicated that the chemical constituents of gongxuening capsules from 10 batches were generally consistent with each other, but the relative content of these constituents varied between batches. The identification and assignment of six polyphyllins in the characteristic chromatograms of the gongxuening capsules were based on the retention time of each standard in the chromatogram of mixed standard compounds.
FIGURE 2: (a) Characteristic chromatograms obtained from 10 batches of gongxuening capsules on Phe-D, and (b) chromatogram of mixed standard compounds on Phe-D.
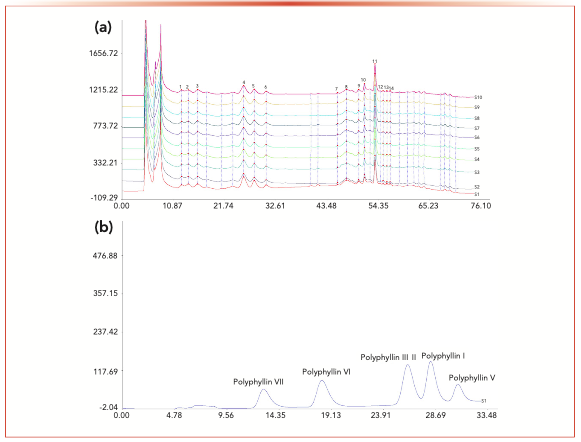
The characteristic chromatograms obtained from 10 batches of gongxuening capsules with DMP-D are shown in Figure 3, and the similarity values are reported in Table I. Among the total 36 peaks, 14 common peaks were observed in all 10 batches. The peak with the retention time 27.989 min, which was identified as polyphyllin I, was chosen as the internal reference peak to calculate the RRT and the RPA of the other peaks. The RSDs of RRT and RPA of other common peaks with respect to polyphyllin I from all samples were in the ranges of 0.14–0.77% and 1.35–59.95%, respectively.
FIGURE 3: (a) Characteristic chromatograms obtained from 10 batches of gongxuening capsules on DMP-D, and (b) chromatogram of mixed standard compounds on DMP-D.
From Figures 2 and 3, it can be seen that six polyphyllins had the same elution order on Phe-D and DMP-D, but had a longer retention time on Phe-D. The unique difference of the two stationary phases was two methyl groups connecting to the phenyl ring on DMP-D; the results showed that the electron density of the phenyl ring affected the retention of analytes.
For the determination of the main polyphyllins in gongxuening capsules, the appropriate amount of six standard substances, polyphyllin I, II, III, V, VI, and VII, was dissolved in methanol to prepare the reference solution at various concentrations. Under the analytical methods, polyphyllin II and III were not separated, thus the content value was determined based on the sum of the two compounds.
The linear equation results of each polyphyllin are as follows: For Phe-D, the linear equations were as follows: y = 1480x − 1088.4 (R2 = 0.9777) for polyphyllin I; y = 724.26x − 566.17 (R2 = 0.9764) for polyphyllin II and III; y = 1014.9x − 930.39 (R2 = 0.9566) for polyphyllin V; y = 908.61x − 33.987 (R2 = 0.9998) for polyphyllin VI; and y = 620.13x − 29.652 (R2 = 0.9998) for polyphyllin VII. For DMP- D, the linear equations were as follows: y = 1438.5x − 195.19 (R2 = 0.9989) for polyphyllin I; y = 755.28x − 217.99 (R2 = 0.9995) for polyphyllin II and III; y = 658.75x − 18.738 (R2 = 0.9999) for polyphyllin V; y = 984.12x − 59.914 (R2 = 0.9999) for polyphyllin VI; and y = 656.61x + 0.0457 (R2 = 0.9999) for polyphyllin VII. Table II shows the recovery rate of six polyphyllins on Phe-D and DMP-D. The data were determined six times. The precision results for each polyphyllin showed that the RSD% were less than 0.84% and 1.02% for Phe-D and DMP-D, respectively, and the RSD% for each polyphyllin in 16 h were less than 1.60% and 1.13% for Phe-D and DMP-D, respectively.
The samples were chosen from 10 batches of gongxuening capsules and tested. The peak area of all polyphyllins was measured five times with each sample and the average value was recorded. The content of each polyphyllin was calculated by the linear equation and the results are illustrated in Table III. As can be observed, the content of polyphyllin varied among different batches of capsules. For example, the content of polyphyllin VI was 0.0724 mg/g in batch 4 while 0.0336 mg/g in batch 6, half of that in batch 4.
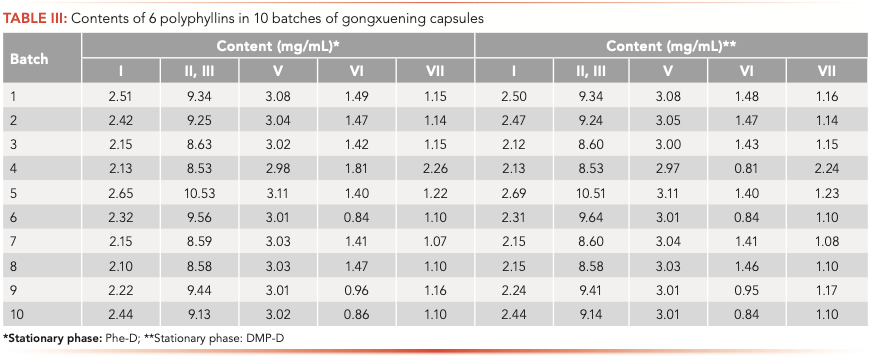
Above all, either the relative content of common peaks, or the content of main polyphyllin, varied between batches. As mentioned above, the quality standard for gongxuening capsules specified in the Chinese Pharmacopoeia is merely based on the content of polyphyllin VI. This quality control method listed in the Chinese Pharmacopoeia seemed insufficient because the content of polyphyllin VI is not sufficient to evaluate the quality of gongxuening capsules. Therefore, a useful approach could be the combination of the typical chromatograms recorded and the determination of multiple evaluation markers. The QC method of the raw material, Paridis rhizoma, is meant to determine the sum of polyphyllins I, II, VI, and VII, which can be the reference for multiple evaluation markers for gongxuening capsules.
Application of Phe-D and DMP-D to the Chiral Separation of Amino Acids
Polyphyllin III contains, in its chemical structure, several asymmetric centers. Therefore, it can be supposed that the two stationary phases have the potential capability of enantioseparation. To test and evaluate the chiral separation capability of the two dioscin stationary phases, 10 native amino acids, namely lysine, leucine, cystine, threonine, serine, valine, alanine, arginine, isoleucine, and histidine, were chosen as chiral analytes. Because of the good water solubility of amino acids, a water-based mobile phase was selected. The mobile phases were investigated considering the type and concentration of the organic additive, and the concentration and the pH value of the buffer to get the best separation results. The enantioseparation results are reported in Table IV and the representative chromatograms are illustrated in Figure 4.
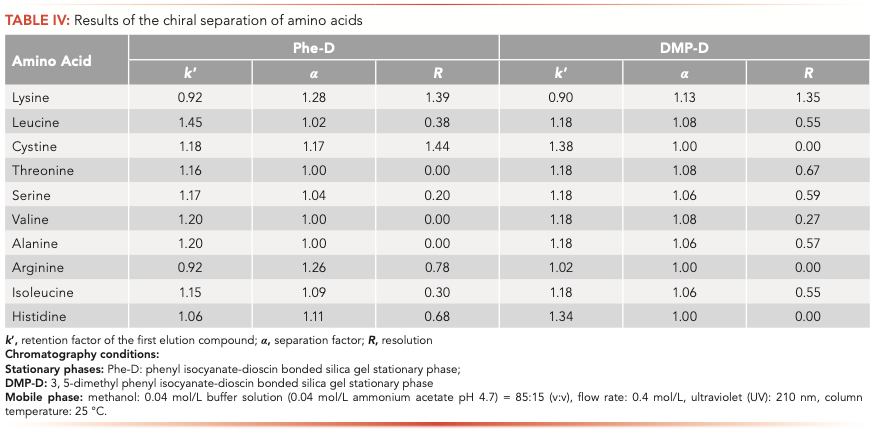
FIGURE 4: (a) The chromatogram of lysine on Phe-D, and (b) the chromatogram of lysine on DMP-D.
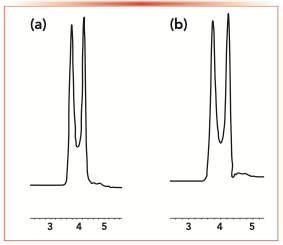
As shown in Table IV, 10 observed amino acid enantiomers saw different degrees of separation, indicating that hydrogen-bonding, and dipole–dipole interactions between the carboxyl (-COOH), amino (-NH2) group and the SP-D benefitted the enatioseparation. Leucine, lysine, serine, and isoleucine enantiomers were resolved using both Phe-D and DMP-D and the chiral separation trend of these three amino acids were similar. Cystine, arginine, and histi- dine were resolved in their enantiomers only on Phe-D, while threonine, valine, and alanine could only be enantioseparated on DMP-D.
Furthermore, from the results listed in Table IV, histidine had a longer retention time on DMP-D (k1 = 1.34) and a shorter retention time on Phe-D (k1 = 1.04). The unique difference of the two stationary phases was two methyl groups connected to the phenyl ring on DMP-D such that the electron density of the phenyl ring of DMP-D is larger. The result showed that π-π stacking between the aromatic ring of histidine and DMP-D affected the retention time. Lysine had the best enantioseparation on both Phe-D and DMP-D (α = 1.28, 1.13), indicating that the hydrogen bonding between the free -NH2 of lysine and SP-D contributed to the resolution, because the free -NH2 can be hydrogen-bonding donor as well as receptor. Lysine had a greater selectivity factor on Phe-D, which may be attributed to the steric structure of the SP-D.
Conclusions
The two dioscin stationary phases can be applied to QC of gongxuening capsules, including qualitative and quantitative analysis, indicating that they have potential applicability for TCM QC. The ingredients of gongxuening capsules had the same elution order on Phe-D and DMP-D, but had a longer retention time on Phe-D. The content of many ingredients varied from batch to batch, so for the QC of the gongxuening capsules, the combination of the typical chromatograms and the multiple evaluation marker determination, is required. In addition, because of the chirality of the two dioscin stationary phases, they showed potential chiral separation ability for amino acids. The 10 observed amino acid enantiomers showed different degrees of separation. The hydrogen-bonding, dipole–dipole interactions, and the π-π stacking and steric effect between the amino acids and the SP-D contributed to the enatioseparation. These results show that two synthesized dioscin stationary phases, Phe-D and DMP-D, exhibited potential applicability for TCM quality control and chiral separation.
Funding
This work was supported by the National Natural Science Foundation of China [number 81102408 and 81860632]; the Yunnan Provincial Science and Technology Department with the Kunming Medical University Applied Basic Research Joint Special Fund Project [number 2017FE468(-172)]; the Science Research Foundation of Yunnan Education Bureau [number 2019J1190]; and the Yunnan Characteristic Plant Polysaccharide Engineering Research Center of Yunnan Province Colleges and Universities (2019).
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals per- formed by any of the authors.
References
(1) A.K. Mallik, H.D. Qiu, M. Takafuji, and H. Ihara, TrAC Trend Anal. Chem. 108, 381–404 (2018).
(2) M. Ganzera and S. Sturm, J. Pharm. Biomed. Anal. 147, 211–233 (2018).
(3) L.L. Li, L.S. Li, and H.R. Yang, Chin. J. Chromatogr. 35(9), 44–49 (2007).
(4) H.M. Chen, L.L. Li, M.F. Liu, and L.F. Huang, J. Nanchang University 33(3), 249–252 (2009).
(5) H.M. Chen, L.L. Li, H.P. Ma, and X.Y. He, Chin. J. Anal. Lab. 29(3), 14–18 (2010).
(6) L.L. Li, X.Q. Chen, H.R. Yang, and Y.M. Wu. Chin, J. Anal. Chem. 35(9), 44–49 (2007).
(7) X.Y. Li, X.J. Gu, K.C. Sun, Y.Y. Yao, and B.C. Shen, Nat. Prod. Res. 27, 780–784 (2015).
(8) Y.Q. Han, X.J. Gu, X.Y. Li, X.L. Chen, L. Wang, and B.C. Shen, Nat. Prod. Res. 26, 268–272 (2014).
(9) X.J. Gu, Y.Q. Han, X.Y. Li, and B.C. Shen, Nat. Prod. Res. 26, 824–829 (2014).
(10) J.M. Padró and S. Keunchkarian, Microchem. J. 140, 142–157 (2018).
(11) G.K.E. Scriba, J. Chromatogr. A. 1467, 56–78 (2016).
(12) B.C. Shen, D.T. Zhang, X.Y. Gu, W. Guo, Y.Q. Han, and X.Z. Xu, Chin. J. Chem. 30, 157–162 (2012).
(13) D.W. Armstrong, Y.B. Tang, S.S. Chen, Y.W. Zhou, and J.R. Chen, Anal. Chem. 66, 1473–1484 (1994).
(14) G.K.E. Scriba, Trend. Trend. Anal. Chem. 120, 115639–115648 (2019).
(15) G.K.E. Scriba, Trend. Anal. Chem. 119, 115628–115636 (2019).
(16) B.C. Shen, J.Y. Yuan, B.J. Xu, and X.Z. Xu, Acta. Chim. Sinica. 67, 2005–2012 (2009).
(17) B.C. Shen, D.T. Zhang, J.Y. Yuan, and B.J. Xu, Chin. J. Chem. 27, 628–632 (2009).
(18) X.F. Tao, L.H. Yin, L. Xu, and J.Y. Peng, Pharmacol. Res. 137, 259–269 (2018).
(19) X.J. Gu, Y.Q. Han, X.Y. Li and B.C. Shen, Nat. Prod. Res. 30, 1454–1460 (2018).
(20) J.H. Zhang, H.L. Xin, Y.M. Xu, Y. Shen, Y.Q. He, H. Ye, B. Lin, H.T. Song, J. Liu, H.Y. Yang, L.P. Qin, Q.Y. Zhang, and J. Du, Ethnopharmacol 213, 230–255 (2018).
(21) L.W. Chen, J. Du, Q.W. Dai, H.C. Zhang, W.S. Pang, and J. Hu, Eur. J. Med. Chem. 83, 294–306 (2014)
(22) S.M. Shan, J.G. Luo, F. Huang, and L.Y. Kong, J. Pharm. Biomed. Anal. 89, 76–82 (2014).
(23) X.N. Wu, H.B. Zhang, S.S. Fan, Y.D. Zhang, Z. Yang, S.M. Fan, P.W. Zhuang, and Y.J. Zhang, Phytomed. 44, 103–108 (2018).
(24) J. Zhao, S.C. Ma, and S.P. Li, J. Pharm. Biomed. Anal. 147, 473–478 (2018).
(25) I. István, P. Antal, and L. Wolfgang, Trend Anal. Chem. 81, 11–22 (2016).
(26) I. István, A. Anita, and P. Antal, J. Chromatogr. A. 1296, 119–139 (2013).
(27) Pharmacopoeia of the People’s Republic of China Edition Vol. 1, 1303–1304 (2015).
Kongchun Sun, Bingquan Chang, Wen Shu, Liangyu Chen, Canyu Yang, and Baochun Shen are with the School of Pharmaceutical Science & Yunnan Key Laboratory of Pharmacology Nature Products at Kunming Medical University in Kunming, China. Xiaojuan Gu is with the School of Pharmaceutical Science at Chuxiong Medical College in Chuxiong, China. Yaqiong Han is with the second Affiliated Hospital of Xinxiang Medical University in Xinxiang, China. Direct correspondence to: shenbaochun@kmmu.edu.cn (Baochun Shen) or yangcanyu@kmmu.edu.cn (Canyu Yang).

New Study Reviews Chromatography Methods for Flavonoid Analysis
April 21st 2025Flavonoids are widely used metabolites that carry out various functions in different industries, such as food and cosmetics. Detecting, separating, and quantifying them in fruit species can be a complicated process.














