Pressure Stability of Wide-Pore Diameter Chiral Chromatography Columns
Analytical columns packed with both 3- and 5- μm polysaccharide-based wide-pore chiral stationary phases (CSP) are shown to be stable to high operating pressures over a prolonged period.
Analytical columns packed with both 3- and 5- μm polysaccharide-based wide-pore chiral stationary phases (CSP) are shown to be stable to high operating pressures over a prolonged period.
In the past, concerns have been expressed about the stability of columns packed with conventional wide-pore silica-based chiral phases to high operating pressures. There is a perception that wide-pore silicas are more fragile than their small-pore counterparts. Consequently, these columns are operated within moderate pressure limits. With the advent of high performance columns based on 3-μm particles, such pressure limits can be restrictive on the development of rapid analytical methods and therefore a re-evaluation of the pressure stability of such phases has been undertaken.
Experimental
Agilent 1100 and 1200 HPLC systems, optimized for minimal extra-column volume, were used for the study. Columns packed with a variety of 3-μm (150 × 4.6 mm) and 5-μm (250 × 4.6 mm) Daicel polysaccharide-based chiral stationary phases (see Table I) were employed. QC testing was carried out using trans-stilbene oxide as solute with 10% 2-propanol in hexane at a flow rate of 0.5 ml/min (5-μm particles) or 1.0 ml/min (3-μm particles). The 3-μm columns were exposed to 200, 280, 360, and 430 bar for one week at each given pressure. Similar tests were carried out for 3-μm reversed phase columns using aqueous acetonitrile as mobile phase. The 5-μm columns were exposed to 360 bar for 10 days followed by 50 h exposure to a cyclic pressure gradient from 90 to 435 bar with a 1 h period.
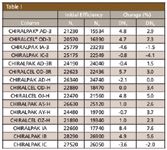
Table I
Results
Table I shows the column efficiency measured before the tests together with the percent change in this parameter for the columns tested. Only very small changes in efficiency were seen, well within the limits of the experimental error over the testing period. No changes in retention or selectivity were seen and the pressure drop across the columns remained constant, indicating that the particles remained undamaged by the procedures. Figure 1 shows the chromatogram for CHIRALPAK AD-3R after the reversed phase pressure stability testing.
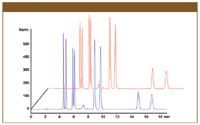
Figure 1
Conclusions
Polysaccharide-based chiral HPLC and SFC columns using wide-pore silica have been shown to be stable to high pressures. These test results, attributable to improvements in silica and packing technologies, allow the operation of both 3- and 5-μm analytical columns at all pressures attainable by conventional HPLC and SFC systems.
CHIRALPAK and CHIRALCEL, AD, AS, OJ, and OD are registered trademarks; IA, IB, IC, AY, and OZ are trademarks of Daicel Chemical Industries, Ltd.

Chiral Technologies, Inc
800 North Five Points Rd, West Chester, PA 19380
tel. 1-800-6-CHIRAL
Website: www.chiraltech.com

SEC-MALS of Antibody Therapeutics—A Robust Method for In-Depth Sample Characterization
June 1st 2022Monoclonal antibodies (mAbs) are effective therapeutics for cancers, auto-immune diseases, viral infections, and other diseases. Recent developments in antibody therapeutics aim to add more specific binding regions (bi- and multi-specificity) to increase their effectiveness and/or to downsize the molecule to the specific binding regions (for example, scFv or Fab fragment) to achieve better penetration of the tissue. As the molecule gets more complex, the possible high and low molecular weight (H/LMW) impurities become more complex, too. In order to accurately analyze the various species, more advanced detection than ultraviolet (UV) is required to characterize a mAb sample.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)














