Peak Purity in Liquid Chromatography, Part III: Using Two-Dimensional Liquid Chromatography
LCGC North America
Here, we see how 2D-LC can solve coelution problems that are difficult or impossible to address using peak purity tools or curve resolution methods.
Is that peak “pure”? How do I know if there might be something hiding under there? This installment explores how two-dimensional (2D) liquid chromatography (LC) can be used to answer that question, particularly to solve coelution problems that are difficult, if not impossible, to address using simple peak purity tools or curve resolution methods.
In part I of this series (1) we discussed how the peak purity tools commonly provided in chromatographic data system software can aid in the detection of impurities in liquid chromatography (LC) analysis. In part II, we continued the discussion by showing how a family of chemometric tools known as multivariate curve resolution (MCR) methods can be used to not only detect the presence of small peaks coeluted with much larger peaks, but also quantify them (2). These methods are particularly useful in cases where the more widely used peak purity tools deployed in commercial software are not sufficiently sensitive to detect low-level impurities in the presence of a larger peak. However, these curve resolution methods also have limitations-namely when the spectral signatures of two (or more) coeluted constituents of the sample are too similar to be resolved effectively by mathematical means. The extreme example of this appears in the case of analyzing a mixture of isomers; here, even mass spectrometric detection is often not sufficiently selective to resolve coeluted compounds.
This month we wrap up this series by describing how two-dimensional (2D) separations can be used to effectively and efficiently solve coelution problems and answer questions about peak purity that are difficult, if not impossible, to address using simple peak purity tools or curve resolution methods. Adding a second dimension of separation is an efficient way to improve the separation power of an LC method in a broad sense (for example, as measured by peak capacity), and to augment the selectivity of an existing separation to tease apart compounds that are otherwise difficult to separate using a single column.
Basic Principles
Here we provide a very brief overview of the concept of 2D separation, and the principles behind the operation of instrumentation used for 2D-LC. Two-dimensional LC provides a suite of tools that are quite powerful, but they do add a level of complexity to the method development. Readers interested in more of the details and deeper discussion are encouraged to refer to a number of recently published resources (3–5), including an overview of recent advances in 2D-LC for biopharmaceutical applications published in LCGC last year (6). Additionally, instrument vendors are now providing instrumentation and guidance for 2D-LC method development, and readers should take advantage of those resources as well.
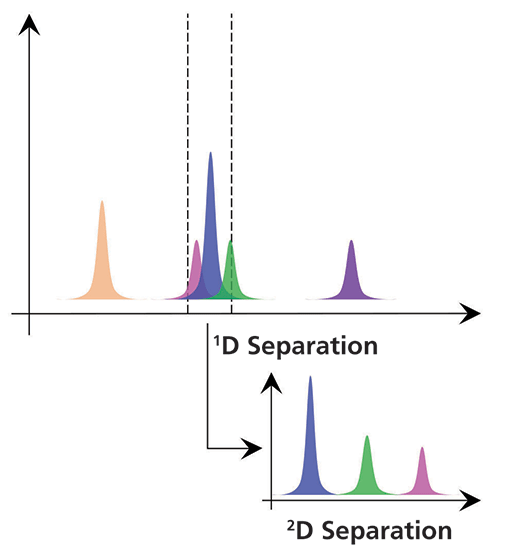
Figure 1: Conceptual view of the value of adding a second dimension of separation to an LC analysis.
A simple view of the value of a second dimension of separation is shown in conceptual form in Figure 1. The idea here is that if our sample is not fully resolved through separation by a first column, we can transfer the portion of the effluent from that first column containing the coeluted analytes to a second column for further separation. Typically, it is very important that the separations by the first dimension (1D) and second dimension (2D) columns be based on different separation principles or mechanisms (for example, hydrophilic-interaction chromatography [HILIC] and reversed-phase chromatography, or reversed phase in both cases, but with one at a low pH and one at a high pH). If we choose the columns and conditions correctly, then there is potential for the mixture of analytes that were coeluted from the first column to be fully resolved by the second column. And, in many cases, this additional separation can be realized without any increase in the total time of analysis.
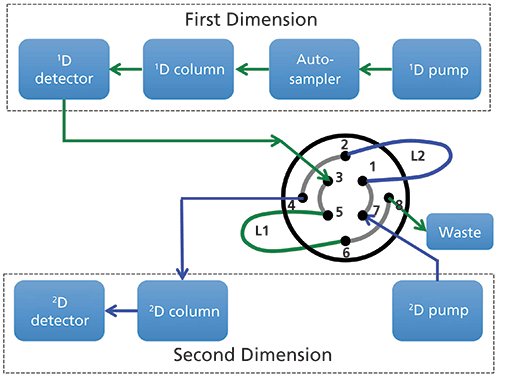
Figure 2: Common configuration of instrument components for 2D-LC analysis.
Figure 2 shows a commonly used configuration of instrument components for 2D-LC analyses. An existing LC system composed of a pump, autosampler, column compartment, and detector is augmented with a second pump, column, and detector, and the two sets of components are connected together with some kind of interface. In this case, the interface shown is a valve that has eight connection ports and two sample loop capillaries (L1 and L2) that can be used to collect effluent from the 1D column and then inject that material into the 2D column for further separation. Figure 3 shows how this valve can be switched between two positions; in Position 1 loop L1 is being filled with effluent from the 1D column while the contents of loop L2 are injected into the 2D column. Some time later the valve can be switched to Position 2; at this point the roles of loops L1 and L2 are reversed and the contents of loop L1 are injected into the 2D column. This kind of interface can be used for either simple heartcutting 2D-LC separations (for example, the kind shown in Figure 1), which is a very focused analysis, and for so-called comprehensive 2D-LC separations where the interface valve switches many times during the analysis to inject many fractions of 1D effluent into the 2D column with the goal of obtaining a more comprehensive separation and view of the constituents of the sample. Other more sophisticated interfaces can be used for hybrid 2D-LC separations known as multiple heartcutting, selective comprehensive, or high resolution sampling 2D separations (3,4,7).
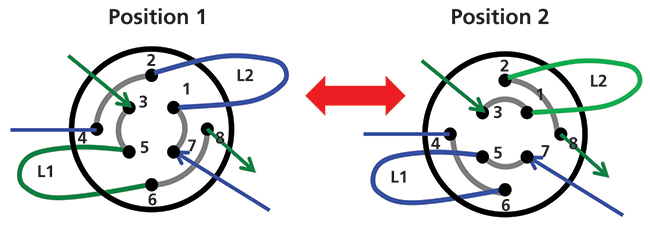
Figure 3: Comparison of flow paths through the interface valve to inject 1D column effluent from either loop L2 (position 1) or loop L1 (position 2) into the 2D column.
Through the following application examples we intend to illustrate how 2D-LC separations can be used to effectively and efficiently solve coelution problems and address questions regarding peak purity, particularly in cases where simple peak purity tools and curve resolution methods may be insufficient.
Application 1: Solving Dynamic Range Problems
Although it is generally important to consider using different column chemistries or conditions for the first and second separations, there are some situations where using the same chemistry in both separations can be quite useful. One such situation is when a much less abundant compound is eluted in the tail (or the front) of a more highly abundant closely eluted compound. Figure 4 illustrates this point by way of simulation. The black trace in Figure 4a is the chromatogram for a two-component mixture where component 1 is present at 2000 times higher concentration compared to component 2 (that is, component 2 is present at 0.05% relative to component 1). We see that component 2 is barely visible as a slight shoulder in the tail of the larger component 1 peak. The red trace shows the chromatogram for component 2 that would be observed if it were injected by itself. So, the problem here is not detecting component 2 per se, but detecting it in the presence of the much more abundant component 1. Now, if we imagine using the interface valve of a 2D-LC system to collect the effluent from the first column from 8.25 to 8.50 min and then inject that fraction into a 2D column operated exactly the same way (that is, same chemistry, dimensions, and flow rate), we would expect to observe the chromatograms in Figure 4b. Now, because we are only transferring about 0.1% of the mass of component 1, the two components are resolved to the point where the quantitation of component 2 could be quite accurate and precise.
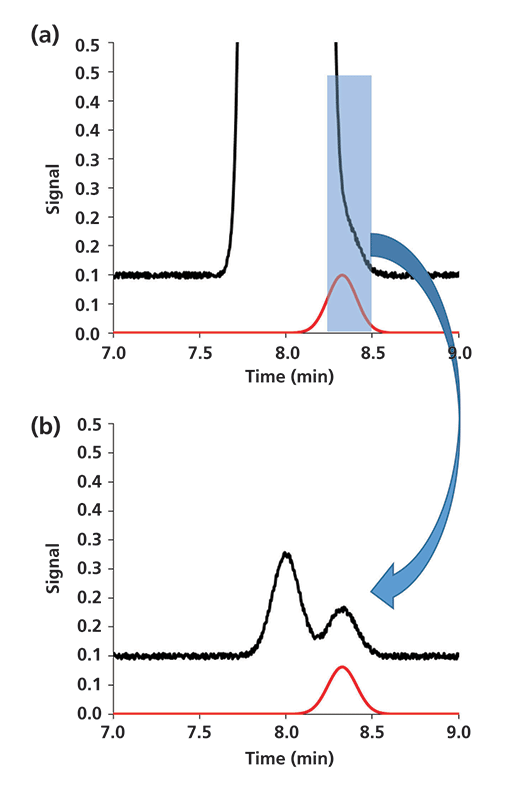
Figure 4: Simulated 2D-LC separation of a two-component mixture using the same column chemistry and conditions in both dimensions: (a) 1D separation, (b) 2D separation. Simulation parameters for both separations: N = 10,000; α = k2/k1 = 1.04; under these conditions the resolution of components 1 and 2 is 1.0.
We use simulation results first here because the conditions for the separation are easily controlled. But, results from the analysis of real samples are always more convincing! Figure 5 shows results of a 2D-LC analysis of a highly concentrated active pharmaceutical ingredient (API) material (8). In this case, a single fraction of 1D effluent from the tail of the API peak was transferred to a 2D column of the same chemistry. In the 2D chromatogram we observe partial separation of the API and impurity because the relative concentrations of the two compounds are more similar (about 10:1) than they were in the original sample. In this case, the small impurity peak could not be detected in the tail of the 1D peak for the API; however, the improved resolution provided by the 2D separation enabled detection and quantitation of the impurity at a much lower concentration than would be possible with a conventional one-dimensional (1D)-LC separation. The LC–mass spectrometry (MS) data (time of flight [TOF]) shows that the impurity peak is a bromo-substituted API (M+1 ion 485.07) instead of the desired chloro-substituted API (M+1 ion 441.12). The bromo-impurity originates from the regulatory starting material (RSM). Structural similarity and reactivity of the impurity (5-bromo-2,4-difluorobenzoic acid) to the RSM (5-chloro-2,4-difluorobenzoic acid) limits the purging of the impurity during the manufacturing process. The only way to limit the level of bromo-API is by controlling the level of 5-bromo-2,4-difluorobenzoic acid in the RSM. The relative level of the bromo-substituted API was less than 0.05% of the API peak, and would not have been detected without 2D-LC separation.
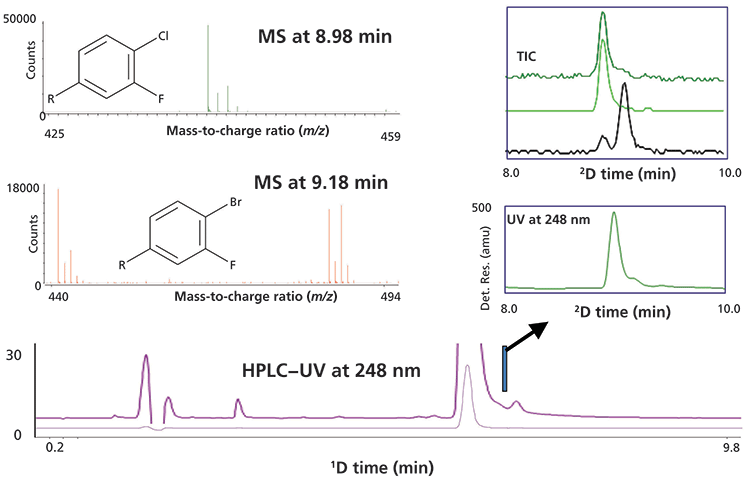
Figure 5: Results from single heartcut 2D-LC analysis of a low-level impurity coeluted in the tail of a highly abundant API peak. In this case the impurity was a bromo-substituted API instead of the desired chloro-substituted API as indicated by the mass spectra for the first and second peak observed in the 2D separation. Adapted with permission from reference 8.
Application 2: Resolving Enantiomers
Experimental results from a second powerful application of single heartcut 2D-LC are shown in Figure 6 (9). Here the sample is first separated using an achiral reversed-phase column (ODS-AQ), and then a single fraction of effluent from the center of the 1D peak is transferred to a 2D column having chiral selectivity for the separation of the enantiomers (Chirobiotic T). The inset chromatogram shows that the enantiomers are well-separated by the 2D column, enabling accurate quantitation of both compounds. In this proof-of-concept separation, the phenylalanine enantiomers are not separated from anything else by the 1D column, but in the case of a real impurity analysis any achiral impurities in the sample could likely be separated by the 1D column before isolating the mixture of enantiomers for further separation by the 2D column. The results of heartcut 2D-LC were comparable to conventional chiral LC with detection limits lower than 0.1%. This 2D-LC approach is now routinely used for simultaneous achiral–chiral analysis of compounds with multiple chiral centers, where developing a chiral 1D-LC method to resolve potential stereoisomers from the desired compound is extremely challenging, if not impractical. As instrument and column technologies have improved, several groups have developed a number of approaches for 2D-LC analysis of mixtures of chiral compounds; for interested readers, there are several excellent examples in the literature (10–16).
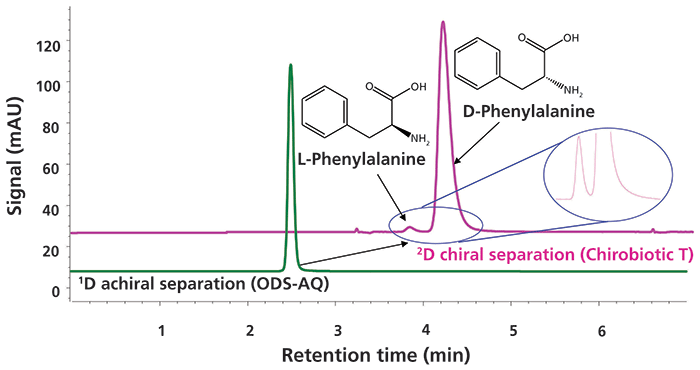
Figure 6: Results from single heartcut 2D-LC separation of the D- and L-enantiomers of phenylalanine. Adapted with permission from reference 9.
Application 3: Addressing Coelution for Very Complex Mixtures
The two example applications discussed above illustrate the potential for 2D-LC to efficiently and effectively resolve relatively simple mixtures. However, the utility of 2D-LC for addressing questions about peak purity and coelution is even more powerful for more complex sample mixtures. An active area of pharmaceutical development that is challenging the capabilities of conventional 1D-LC is the development of antibody–drug conjugates (ADCs) as drug substances. ADCs are assembled by conjugating a cytotoxic small molecule to an antibody protein using a low-molecular-weight linker molecule. Analysis of these linkers is challenging because of the complex routes involved in their synthesis and their relatively high reactivity. Figure 7 shows a selective comprehensive 2D-LC separation of a purified linker intermediate, where 10 fractions of 1D column effluent collected over the entire 1D linker peak are transferred one at a time to the 2D column for further separation. Figure 7a is focused on the region of the 1D chromatogram dominated by the API, and shows the fractionation of this peak into 10 fractions starting at 11.15 min. Figure 7b shows the resulting 2D chromatogram obtained from these 10 fractions as a contour plot. The locations of three impurity peaks (A–C) that were coeluted with the API in the first dimension, but are nicely resolved by the 2D separation, are indicated with yellow ellipses. Finally, Figure 7c shows a single 2D chromatogram corresponding to the separation of fraction 5, which more clearly shows the separation of the three impurities from the API. Because the three impurities were coeluted with the 1D linker peak, they are not visible in the 1D chromatogram. Here again, 2D-LC provides an efficient route to dramatically increase the selectivity of the overall analysis through the addition of a 2D separation. In this case, choosing a 2D column with selectivity complementary to that of the 1D column was important for achieving the separation shown here.
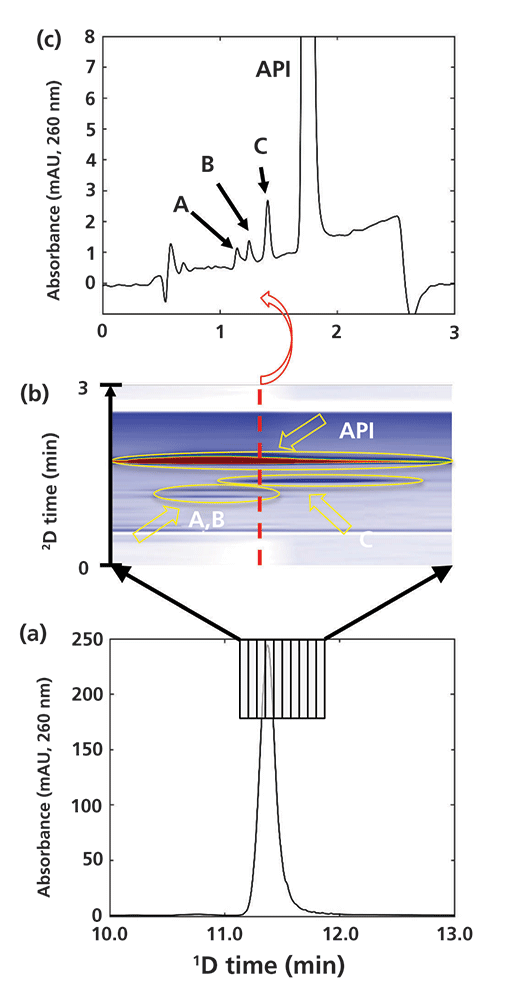
Figure 7: Selective comprehensive 2D-LC separation focused on detection of impurities coeluted from the 1D column with a drug linker intermediate molecule used in the synthesis of an antibody–drug conjugate (ADC).
Closing Remarks
In this three-part series, we discussed different approaches to addressing questions about peak purity and coelution. These include peak purity algorithms that are based on the evolution of spectra across a chromatographic peak, curve resolution methods that are designed to mathematically resolve analyte peaks that are not resolved chromatographically, and finally two-dimensional separations. Each approach has strengths and weaknesses, and they vary tremendously in terms of ease of use, cost of implementation, and so on. It should be pointed out that the peak purity and curve resolution tools described in parts I and II of this series can also be applied to 2D chromatography data as well, enhancing the power of these methods even more (1,16). We hope that this series of "LC Troubleshooting" installments will help readers consider which approach might be most useful for them in particular analytical situations they face now and in the future.
References
(1) S.C. Rutan, C.J. Venkatramani, and D.R. Stoll, LCGC North Am. 36(2), 100–111 (2018).
(2) D.W. Cook, S.C. Rutan, C.J. Venkatramani, and D.R. Stoll, LCGC North Am. 36(4), 248–255 (2018).
(3) D. Stoll, in Handbook of Advanced Chromatography /Mass Spectrometry Techniques, M. Holcapek and W.C. Byrdwell, Eds. (Elsevier, London, 2017), pp. 227–286.
(4) D.R. Stoll and P.W. Carr, Anal. Chem.89, 519–531 (2017).
(5) B.W.J. Pirok, A.F.G. Gargano, and P.J. Schoenmakers, J. Sep. Sci. 41(1), 68–98 (2017).
(6) D.R. Stoll and T.D. Maloney, LCGC North Am. 35(9), 680–687 (2017).
(7) D.R. Stoll, K. Zhang, G.O. Staples, and A. Beck, in Advances in Chromatography (CRC Press, Boca Raton, Florida, 2018), in press.
(8) C.J. Venkatramani, J. Girotti, L. Wigman, and N. Chetwyn, J. Sep. Sci. 37, 3214–3225 (2014).
(9) C.J. Venkatramani, L. Wigman, K. Mistry, and N. Chetwyn, J. Sep. Sci. 35, 1748–1754 (2012).
(10) M. Peñín-Ibáñez, M.J. Santos-Delgado, and L.M. Polo-Díez, Anal. Bioanal. Chem. 409, 1135–1144 (2017).
(11) C.L. Barhate, E.L. Regalado, N.D. Contrella, J. Lee, J. Jo, A.A. Makarov, D.W. Armstrong, and C.J. Welch, Anal. Chem. 89, 3545–3553 (2017).
(12) C.J. Venkatramani, M. Al-Sayah, G. Li, M. Goel, J. Girotti, L. Zang, L. Wigman, P. Yehl, and N. Chetwyn, Talanta 148, 548–555 (2016).
(13) E.L. Regalado, J.A. Schariter, and C.J. Welch, J. Chromatogr. A 1363, 200–206 (2014).
(14) Q. Liu, X. Jiang, H. Zheng, W. Su, X. Chen, and H. Yang, J. Sep. Sci. 36(9), 3158–3164 (2013).
(15) Y. Yang, N. Rosales-Conrado, V. Guillén-Casla, M.E. León-González, L.V. Pérez-Arribas, and L.M. Polo-Díez, Chromatographia 75, 1365–1375 (2012).
(16) C. Lee, J. Zang, J. Cuff, N. McGachy, T.K. Natishan, C.J. Welch, R. Helmy, and F. Bernardoni, Chromatographia 76, 5–11 (2012).
ABOUT THE AUTHORS
Dwight R. Stoll

Dwight R. Stollis the editor of "LC Troubleshooting." Stoll is an associate professor and co-chair of chemistry at Gustavus Adolphus College in St. Peter, Minnesota. His primary research focus is on the development of 2D-LC for both targeted and untargeted analyses. He has authored or coauthored more than 50 peer-reviewed publications and three book chapters in separation science and more than 100 conference presentations. He is also a member of LCGC's editorial advisory board. Direct correspondence to: LCGCedit@ubm.com
Sarah C. Rutan

Sarah C. Rutanis a professor of chemistry at Virginia Commonwealth University (VCU), in Richmond, Virginia, where she has been on the faculty for 33 years. Her research spans a broad range of areas in analytical chemistry and chemometrics, and is currently focused on the development of chemometric methods for improving chromatographic analyses, especially comprehensive 2D chromatography. She has more than 100 publications and numerous presentations on these topics.
C.J. Venkatramani

C.J. Venkatramani is a senior scientist at Genentech USA and has more than 15 years experience in the pharmaceutical industry. He was the key member of Genentech technical team instrumental in the successful launch of gRed's first small molecule Erivedge, leading from development to commercial. His areas of interest include multidimensional chromatography and ultratrace analysis of genotoxic impurities.

Troubleshooting Everywhere! An Assortment of Topics from Pittcon 2025
April 5th 2025In this installment of “LC Troubleshooting,” Dwight Stoll touches on highlights from Pittcon 2025 talks, as well as troubleshooting advice distilled from a lifetime of work in separation science by LCGC Award winner Christopher Pohl.
Mobile Phase Buffers in Liquid Chromatography: A Review of Essential Ideas
December 11th 2024In this installment of "LC Troubleshooting," Dwight Stoll discusses several essential principles related to when and why buffers are important, as well as practical factors, such as commonly used buffering agents, that are recommended for use with different types of detectors.
In-Loop Analyte Degradation in Two-Dimensional Liquid Chromatography: Example and Solutions
September 13th 2024In this installment of “LC Troubleshooting,” we describe an artifact that can arise because of compound degradation during the transfer of fractions of the first dimension (1D) column effluent to the 2D separation.







