Multi-Channel SFC System for Fast Chiral Method Development and Optimization
Special Issues
According to the FDA policy statement, developing stereoisomeric drugs, each enantiomer should be evaluated.
APPLICATION BENEFITS
The Method Station X5 SFC System offers parallel screening for method development and further optimization in single channel mode, on one platform, for chiral separations by SFC. The proprietary plumbing enables software controlled switching between parallel and single channel mode without the need for any hardware change.
WATERS SOLUTIONS
Method Station X5 SFC System
2998 Photodiode Array (PDA) Detector
2489 UV/Visible Detector
SuperChrom™ Software
KEYWORDS
SFC
Chiral chromatography
Chiral stationary phase
Method development
Parallel screening
INTRODUCTION
According to the FDA policy statement, developing stereoisomeric drugs, each enantiomer should be evaluated.1 As a result, the pharmaceutical industry has escalated its emphasis on the generation of enantiomerically pure compounds before undertaking pharmacokinetic, metabolic, physiological, and toxicological evaluations.2 Chiral chromatography, especially SFC, is the most widely used technique for obtaining milligrams to multi-grams of pure enantiomers in drug discovery. In SFC, supercritical CO2 combined with polar organic solvent(s), most commonly methanol, are used as the mobile phase. Due to the higher diffusivity and lower viscosity of supercritical fluid, SFC often provides a 3- to 8-fold faster separation than normal-phase HPLC. For chiral purification, SFC also offers significant cost savings by reducing organic solvent usage and removal as well as the time and energy required post-purification. As SFC instrumentation continues to improve, it is gradually overtaking HPLC as the first choice for chiral separations and purification.
At the drug discovery stage, it is important to minimize the time required to obtain pure enantiomers, from milligrams to multi-grams. With stacked injections, such quantities can be obtained using SFC in a matter of hours. However, stacked injections can only be performed under isocratic conditions. Consequently, users often start method development with a screening of multiple chiral stationary phases (CSPs) using a generic gradient and finish the optimization with an isocratic method for scale-up.
Despite the speed advantage SFC can offer, method development still remains one of the major bottlenecks for chiral separations by SFC. Due to the lack of a universal CSP, trial-and-error screening of a set of CSPs has been the predominant approach to chiral method development. Currently, most commercial instruments employ automated column and solvent switching to facilitate this screening process. However, it is time-consuming because each column has to be tested individually in a temporal manner. To circumvent this problem, multi-column, parallel screening approaches in both LC3 and SFC4 have been proposed. Typically, an injected sample was carried by the mobile phase and divided into multiple columns with varying detection schemes. The extent of improvement in throughput depends upon the number of channels the main flow splits. These proposed approaches, however, suffer from the lack of independent control of multi-channel and single-channel required by method development and optimization. After the parallel screening, users would have to either re-plumb the system to the single channel configuration or resort to the use of another dedicated single channel system to perform isocratic optimization.
In this application note, we introduce the first commercially available parallel SFC system, Method Station X5 SFC System, from its design objectives to operation using 4-benzoyloxy-2-azetidinone as a model compound.
EXPERIMENTAL
The Method Station X5 SFC System consists of a Fluid Delivery Module (FDM), Alias Autosampler, Analytical-2-Prep™ Column Oven, Automated Back Pressure Regulator (ABPR), 2998 PDA Detector, and four 2489 UV/Visible detectors. SuperChrom Software was used for data acquisition and analysis. In the current study, each injection is split into five individual channels. All columns were purchased from commercial resources.
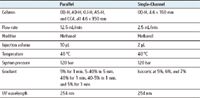
Figure 1 shows 4-benzoyloxy-2-azetidinone, obtained from Sigma Aldrich (St. Louis, MO, USA). A stock solution of 2.5 mg/mL was prepared by dissolving the compound in methanol. Key experimental parameters are listed as follows:
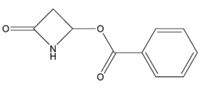
Figure 1. The chemical structure of 4-benzoyloxy-2-azetidinone.
RESULTS AND DISCUSSION
Despite the lack of one universal column for chiral separations by SFC, it is the experience of many users that more than 75% of the pharmaceutical relevant chiral molecules can be separated by one or multiple of the following CSPs: OD-H, AD-H, OJ-H, and AS-H.5 The advancements in CSPs, especially the newly commercialized IC (Chiral Technologies Inc., West Chester, PA) and Cellulose-LUX-2 (Phenomenex, Torrance, CA), have further improved the success rate of chiral separations by SFC. The Method Station X5 SFC System is designed to primarily deal with those chiral molecules that can be separated using the aforementioned four to six CSPs, to quickly select the appropriate CSP in a parallel format, and to optimize the isocratic condition in a single channel mode for ensuing purification. The proprietary plumbing of the Method Station X5 SFC System enables a software controlled switching between parallel and single channel mode without any hardware change.
Figure 2 shows the SFC chromatograms of 4-benzoyloxy-2-azetidinone obtained in parallel screening mode. Both AD-H and AS-H columns yielded baseline resolution of the enantiomers. OD-H generated partial separation. OJ-H and CC4 offered no resolution. Based on the screening results, the system should then be switched to the single channel mode with either AD-H or AS-H for an optimal isocratic method for scale-up. For illustration purposes, we selected the OD-H column instead, for further method optimization to mimic a common scenario where only partial separation is achieved with a gradient in the parallel mode. Based on the chromatograms shown in Figure 2, we ran a sequence consisting of three methods with 7%, 6%, and 5% modifier respectively.

Figure 2. SFC chromatograms of 4-benzoyloxy-2-azetidinone obtained in parallel mode.
The results are shown in Figure 3. In this particular experiment, the 5% isocratic method appears to yield the best resolution.

Figure 3. SFC chromatograms of 4-benzoyloxy-2-azetidinone obtained under isocratic conditions using various volumes of methanol.
It is noted that the Method Station X5 SFC System is applicable for achiral method development and optimization as well, as long as a limited number of columns can be selected to encompass a sufficiently wide selectivity range.
CONCLUSIONS
The Method Station X5 SFC System offers a streamlined process for SFC method development and optimization. The proprietary plumbing enables a software controlled switching between parallel and single channel mode. The system will expedite the method development by five-fold; hence, shortening the turnaround time for those molecules that can be separated by the commonly used CSPs.
References
1. FDA's Policy Statement for the Development of New Stereoisomeric Drugs May 1, 1992; Corrections made on January 3, 1997. http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm122883.htm
2. Zhang Y, Wu D, Wang-Iverson DB, and Tymiak A. A. Drug Discov. Today, 2004; 10 (8): 571-577.
3. Zhang Y, Watts W, Nogle L, and McDonnell O. J. Chromatogr. A., 2004; 1049: 75-84.
4. Zeng L, Xu R, Laskar DB, and Kassel DB. J. Chromatogr. A., 2007; 1169: 193-204.
5. Francotte ER. J. Chromatogr. A., 2001; 906: 379-397.
Waters Corporation
34 Maple Street
Milford, MA 01757 U.S.A.
T: 1 508 478 2000
F: 1 508 872 1990

Regulatory Deadlines and Supply Chain Challenges Take Center Stage in Nitrosamine Discussion
April 10th 2025During an LCGC International peer exchange, Aloka Srinivasan, Mayank Bhanti, and Amber Burch discussed the regulatory deadlines and supply chain challenges that come with nitrosamine analysis.











