Basic Principles for Purification Using Supercritical Fluid Chromatography
Special Issues
Although supercritical fluid chromatography (SFC) is not a new technique, preparative SFC is becoming increasingly more popular with advances in instrumentation, software and chemistry.
APPLICATION BENEFITS
Basic principles for the separation of compounds from mixtures using supercritical fluid chromatography (SFC) are similar to the fundamentals of preparative liquid chromatography.
- Fast, large-scale separations increase throughput
- DMSO can be used for samples with limited solubility
- Different column selectivities improve compound isolation
- Column scalability ensures reproducible chromatograms at the analytical and preparative scales
WATERS SOLUTIONS
Waters Prep 100 SFC UV Directed System
Waters Method Station SFC System
Viridis™ SFC Columns
Optimum Bed Density (OBD™) Technology
KEY WORDS
Supercritical fluid chromatography
Viridis SFC columns
Preparative SFC
OBD technology
INTRODUCTION
Although supercritical fluid chromatography (SFC) is not a new technique, preparative SFC is becoming increasingly more popular with advances in instrumentation, software and chemistry. Basic principles for isolating compounds with SFC are similar to the fundamental rules for large-scale preparative liquid chromatography. In this study, we illustrate the effect of flow rate, sample dissolution solvent, and column selectivity on the isolation of compounds from a mixture. Columns packed with Optimum Bed Density (OBD) Technology1 and with matching chemistry ensure easy and predictable scalability from the small scale to larger preparative separations.
EXPERIMENTAL
Sample preparation
Compound mixture 1: Coumarin, ibuprofen, and carbamazepine; 20 mg/mL each in methanol
Compound mixture 2: Ibuprofen and carbamazepine; 20 mg/mL each in methanol
Compound mixture 3: Ibuprofen and carbamazepine; 20 mg/mL each in DMSO (dimethyl sulfoxide)
Compound mixture 4: 10 mg/mL Fenoprofen, 15 mg/mL Ketoprofen, 20 mg/mL Flavone, 20 mg/mL Ibuprofen, and 20 mg/mL Coumarin in methanol
Experimental conditions
Prep SFC system: Waters Prep 100 SFC UV
Detection: Waters 2998 Photodiode Array
Columns: Viridis SFC 2-Ethylpyridine, 4.6 x 150 mm, 5 μm, Part Number 186004937
Viridis SFC 2-Ethylpyridine OBD, 19 x 150 mm, 5 μm, Part Number 186004945
Viridis SFC Silica OBD, 19 x 150 mm, 5 μm, Part Number 186004918
Viridis SFC 2-Ethylpyridine OBD, 19 x 100 mm, 5 μm, Part Number 186004944
Column temperature: 40 °C
Injection volume: Reported in figures
Flow rate: Reported in figures
Co-solvent: Methanol
Gradient: Reported in figures
Analytical SFC system: Waters Method Station SFC System
RESULTS AND DISCUSSION
Flow Rate
The flow rate used in preparative LC separations is limited by a number of factors including the length and diameter of the column, the particle size, and the system backpressure. Low viscosity carbon dioxide, the main component of the mobile phase in SFC, enables the use of higher flow rates than those generally used in liquid chromatography. The backpressure associated with column length is also lower, enabling usage of longer columns to increase resolution. Higher flow rates increase throughput by reducing the amount of time to complete a separation. As shown in Figure 1, the chromatographic profile is the same at different flow rates provided that the slope of the gradient expressed in percent change per column volume remains constant. This is accomplished by decreasing the run time in the same proportion as the flow rate is increased.
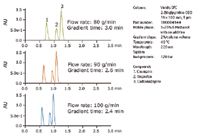
Figure 1. Compound mixture 1 run with constant gradient slope at 80, 90, and 100 g/min. The gradient separation time decreases with increased flow rate while maintaining resolution.
Sample Dissolution and Loading Solvent
Isolating compounds from mixtures requires that the sample be completely soluble before injection. Methanol, the most common co-solvent in SFC, does not solubilize all samples at the high concentrations usually required for typical preparative separations. Large volumes of solvents like methanol can limit the mass capacity of the column due to strong solvent effects that can distort the chromatography. Modifier stream injection, the standard patented configuration of all Waters Prep SFC systems, was designed to reduce this solvent effect. Dimethyl sulfoxide (DMSO) is used regularly in purification labs to solubilize many different types of compounds. Figures 2 and 3 illustrate how DMSO injections in SFC can result in higher loading and better resolution for achiral applications as compared to samples injected in methanol.
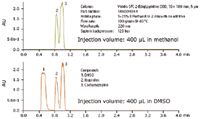
Figure 2. The resolution between the compounds dissolved in DMSO is greater than for the same size injection with the compounds dissolved in methanol.
Note: For chiral applications, caution should be considered as DMSO can severely damage a traditional coated chiral stationary phase. If DMSO should be required for sample solubility, an immobilized chiral column should be selected.
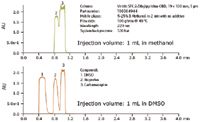
Figure 3. The difference in resolution between the sample mixture dissolved in methanol and in DMSO is more pronounced at a 1 mL injection volume. Better resolution improves the mass loading leading to higher purity and increased purification process efficiency.
Selectivity
In SFC, multiple columns are routinely tested to determine which provides the best resolution and peak shape for components in a mixture. Good resolution between compounds leads to isolations of target compounds with high purity. In the example below, the 2-ethylpyridine column shows excellent separation of all five compounds in the sample mixture. The same sample mixture analyzed on a silica column shows only three component peaks because it has a different selectivity. Coumarin and ibuprofen coelute at about 0.8 minutes and flavone and fenoprofen coelute slightly later. Flavone and ibuprofen also change elution order on the silica column. Ketoprofen is well-separated from all of the other peaks on both columns.

Figure 4. The selectivity difference between the 2-ethylpyridine and silica columns illustrates the effect that column chemistry can have on the separation.
Scaling
Crude sample mixtures are usually analyzed with a screening gradient that starts from a low percentage of organic solvent and ends at a higher percentage of organic solvent in a relatively short time. If the compounds of interest are well resolved, the gradient may be scaled directly for purification. When all compounds elute before the completion of the gradient and are fully resolved, a simple reduction in the length of the gradient is acceptable. The fast flow rates used in SFC combined with modified gradients considerably shorten the total time required for purification of target compounds.

Figure 5. A loading study on the analytical column determines how much mass can ultimately be injected on the preparative column. Shown here, the increasing peak width is a result of the solvent effect associated with larger injection volumes.
Scaling separations requires matching column chemistry and particle size as well as appropriately scaled injection volumes and gradients. Waters preparative SFC columns are packed with OBD Technology, ensuring excellent bed stability and comparable performance to Waters analytical SFC columns. OBD preparative columns are packed to bed densities which closely match the equivalent analytical column. This innovative procedure produces preparative columns with excellent stability, reproducibility, and efficiency.

Figure 6. Preparative chromatography scaled from the analysis completed on the small scale column. Slight differences in retention time are related to the differences in the plumbing configuration and dwell volumes between the analytical and preparative systems.
A loading study is performed at the small scale to determine how much sample mass can be loaded on the column. The goal is to maximize the load without compromising the resolution. Improved peak resolution leads to higher loads and better purity for isolated compounds. Higher column loading reduces the number of runs required for obtaining enough material for subsequent experiments. The chromatography from the loading study is depicted in Figure 5. The conservative 35 μL injection volume shows good resolution between all of the compounds in the mixture and is subsequently scaled for the preparative run on the large column. A total volume of 600 μL was injected on the 19 x 150 mm preparative SFC column. Figure 6 compares the chromatography using the small scale modified gradient at maximum load with the large-scale chromatography used for the isolation. The selectivity is identical, a critical factor when using analytical chromatography as a scouting tool for isolation and purification. The differences in retention time and resolution between the analytical and preparative chromatograms can be attributed to factors including system volume and injection mode. In particular, the patented modifier stream injection mode* is employed for all Waters preparative SFC systems, minimizing the solvent effect and therefore improving throughput and efficiency.
* US Patent #6,576,125
CONCLUSIONS
- The basic principles of liquid chromatography can be applied to supercritical fluid chromatography (SFC).
- Supercritical fluid chromatography can be run at increased flow rates making isolations faster and increasing throughput.
- Samples with limited solubility can be dissolved in dimethyl sulfoxide and purified using SFC. Using DMSO for sample dissolution makes the purification process easier by reducing the uncertainty associated with sample solubility.
- Resolution and loading of samples dissolved in DMSO can be improved when compared to the same samples dissolved in methanol. Better sample resolution leads to products with higher purity. Higher loading improves the efficiency of the purification process.
- Column selectivity impacts the separation of mixture components. Screening different columns with fast gradients leads to a decision about which preparative column to use for isolating the compounds in the sample.
- The ability to scale between analytical and preparative OBD columns leads to identical separations at both the small and large scales, provided that the injection volume, slope, and gradient time are scaled correctly and the chemistry and particle size remain constant. Column scalability removes uncertainty in peak identification, thus facilitating higher purification process efficiency.
References
1. Optimum Bed Density (OBD) Columns: Enabling Technology for Laboratory-Scale Isolation and Purification. Waters White Paper 720001939EN 2006 Nov.
Waters Corporation
34 Maple Street
Milford, MA01757U.S.A.
T: 1 508 478 2000
F: 1 508 872 1990

New Method Explored for the Detection of CECs in Crops Irrigated with Contaminated Water
April 30th 2025This new study presents a validated QuEChERS–LC-MS/MS method for detecting eight persistent, mobile, and toxic substances in escarole, tomatoes, and tomato leaves irrigated with contaminated water.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)









