Matrix Elimination- Inline Removal of Chloride Using InGuard Ag and Na Cartridges
In ion chromatography, the presence of a large amount of matrix ions makes quantification of the target ions difficult.
Bernard G. Sheldon1 , Rida Al-Horr2 , and Rosanne Slingsby1 , 1 Dionex Corporation, 2 Dow Chemical
In ion chromatography, the presence of a large amount of matrix ions makes quantification of the target ions difficult. Selective removal of matrix ions–matrix elimination–can be performed by treating a sample with a solid-phase extractant. Halides can be removed by precipitation with silver, which is present as a counterion in a cation-exchange resin. A subsequent treatment with a cation-trapping column removes residual dissolved silver ions.
When analyzing a sample for nitrite, it is important to maintain a high pH to preclude oxidation of nitrite to nitrate, which occurs readily at low pH. Therefore, sodium ion (Na+ ) should be used as the counterion on the silver-trapping column.
Solid-phase manual extraction columns, such as OnGuard® II cartridges are useful for processing a limited number of samples; they are discarded after use with a single sample. Newly introduced InGuard™ cartridges provide automated inline matrix elimination capabilities, which reduce sample-processing costs, increase processing reliability, and enhance ease-of-method documentation and transfer.
InGuard cartridges are used in an ion chromatograph to treat samples before they are injected. The sample is metered with a sample loop and transferred to a concentrator column after being passed through the InGuard cartridge or cartridges. While InGuard cartridges have capacities similar to their OnGuard analogues, the InGuard cartridges will only be exposed to a small amount of sample per injection, and therefore can be used many times before they are exhausted.
Experimental
A Dionex ICS-3000 system with a DP dual pumping system and a Detector Compartment equipped with two 6-port valves was used here.
The first valve was a standard high-pressure 6-port injection valve equipped with a 100 µL sample loop. The second valve was a 6-port high-pressure valve installed on the Automation Manager of the DC. An InGuard Ag and an InGuard Na cartridge were plumbed in series between the two valves. The second pump transferred the sample from the sample loop through the cartridges and onto a concentrator column (Figure 1).
The passage of the sample through the InGuard cartridges may cause some dilution and band-broadening of the sample. A concentrator column traps and concentrates the sample into a small volume before injection onto the analytical column.
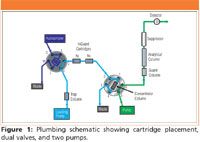
Figure 1
Results and Discussion
This application demonstrates the utility of a fully automated method with new InGuard Ag and Na inline matrix elimination cartridges for removal of chloride from a sample before injection. If not removed, a 1.6% sodium chloride sample, as the one being analyzed, would overwhelm the capacity of the analytical column used. The result would be an offscale peak with no resolution of the analytes of interest (Figure 2).
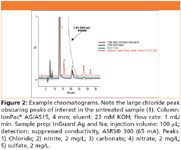
Figure 2
InGuard Ag cartridges contain approximately 5 mEq of silver ion, which is sufficient to remove the chloride from approximately 100 injections of 100 µL each of a 1.6% sodium chloride solution.
OnGuard, IonPac, and ASRS are registered trademarks and InGuard is a trademark of Dionex Corporation.

Dionex Corporation
1228 Titan Way, P.O. Box 3603, Sunnyvale, CA
tel. (408)737-0700; fax (408)730-9403
Website: www.dionex.com

The Benefits of Custom Bonded Silica
April 1st 2025Not all chromatography resins are created equal. Off-the-shelf chromatography resins might not always meet the rigorous purification requirements of biopharmaceutical manufacturing. Custom bonded silica from Grace can address a wide range of separation challenges, leading to real performance improvements. Discover more about the latest innovations in chromatography silica from Grace, including VYDAC® and DAVISIL®.
5 Things to Consider When Selecting a Chromatography Silica
April 1st 2025Particularly in the pharmaceutical industry, drug purity isn’t just a goal – it’s essential for achieving safety, stability and efficacy. However, purification is easier said than done, especially with challenging molecules like DNA and RNA “oligonucleotides,” due in large part to their diversity and the range of impurities that can be generated during production. Enter DAVISIL® chromatographic silica, with a wide range of pore diameters and particle sizes to meet your specific application, performance and sustainability requirements. Before you choose the chromatography resin for your next purification application, take a look at these 5 considerations.
Automating Protein Purification: Efficiency, Yield, and Reproducibility
March 27th 2025Recent advancements in automated protein purification stress the importance of efficiency, scalability, and yield consistency. This eBook compares different purification platforms, highlighting their impact on downstream applications and demonstrating how automation enhances throughput and process control.
MilliporeSigma: Ultrapure Water for Sensitive LC-MS Analysis of Pesticides
March 25th 2025The aim of the study was to illustrate the efficiency of Milli-Q® water purification systems in eliminating pesticides from tap water, thereby producing and delivering reliable and consistent-quality ultrapure water suitable for pesticides analysis















