Light Scattering for the Masses Liposome Characterization by FFF-MALS-QELS
Wyatt Application Note
Liposomes are made of lipid bilayers and are often used in drug delivery by encapsulating the core with therapeutic drugs. During liposome research, formulation, manufacturing and quality control, it is of great importance to monitor liposome size and encapsulation. Field-flow fractionation (FFF) with the concomitant use of Multi-Angle Light Scattering (MALS) and Quasi-Elastic Light Scattering (QELS, aka dynamic light scattering) is an ideal tool for such characterization.
Here, we report the analytical results of two liposome samples, one empty and one filled. Using the Eclipse FFF system followed by a DAWN HELEOS (with embedded WyattQELS instrumentation), the FFF method was optimized with the aid of Wyatt ISIS FFF simulation software. The online QELS directly measures the hydrodynamic radius, Rh, whereas the HELEOS measures the root-mean square radius, Rg.
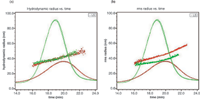
Figure 1: Hydrodynamic radius (a) and root-mean square radius (b) plotted against elution time overlaid with 90° LS signals for empty liposome sample (red) and filled liposome sample (green). The R h and R g values are determined by the respective QELS and MALS detectors. The results from duplicate runs of each sample are shown here to demonstrate the reproducibility of the FFF-MALS-QELS analysis.
The WyattQELS detector was placed at approximately 143° in order to extend the Rh measurement up to 300 nm. Both Rg and Rhare plotted against elution time in Figure 1. The results from duplicate runs demonstrate excellent reproducibility of the FFF-MALS-QELS method. Figure 1 also shows that the Rh values for both empty and filled liposomes are well overlaid, suggesting the separation is based on hydrodynamic radius as expected from an FFF separation. However, Rg values for these two liposomes do not overlay, which indicates these two liposomes have different degrees of encapsulation.
Root-mean square radii, Rg, were then plotted against hydrodynamic radii, Rh, for these two liposomes. The slope of Rg vs Rh plot yields the internal structure of the liposomes. The empty liposome sample has a slope of 1.0, consistent with a spherical shell structure. The filled liposome sample, on the other hand, has a slope of 0.75, in good agreement with that of a solid sphere structure of uniform density.
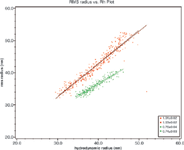
Figure 2: Root-mean square radius, R g, plotted against hydrodynamic radius, R h, for empty liposome sample (red) and filled liposome sample (green). The slopes for empty and filled liposomes are 1.0 and 0.75, respectively.
For liposomes or other nanoparticles, FFF-MALS-QELS provides an easily adaptable yet powerful characterization tool to obtain information on particle size, size distribution, particle count, as well as structure — all without making assumptions about the particles or their composition.
DAWN, miniDAWN, ASTRA, Optilab and the Wyatt Technology logo are registered trademarks of Wyatt Technology Corporation.

Wyatt Technology Corp.
6300 Hollister Avenue, Santa Barbara, California 93117, USA
tel: +1 805 681 9009 fax: +1 805 681 0123
E-mail: info@wyatt.com Website: www.wyatt.com

Separating Impurities from Oligonucleotides Using Supercritical Fluid Chromatography
February 21st 2025Supercritical fluid chromatography (SFC) has been optimized for the analysis of 5-, 10-, 15-, and 18-mer oligonucleotides (ONs) and evaluated for its effectiveness in separating impurities from ONs.


















