Analysis of Cholesterol Lowering Drugs (Statins) Using Dried Matrix Spots Technology and Poroshell 120 HPLC Columns
Agilent Application Note
Introduction
Dried blood spot (DBS) technology combined with the analytical capability of modern mass spectrometers (LC–MS–MS) has emerged as an important method for the quantitative bioanalysis of small molecules.
Four statins were analysed on novel non-cellulose based Dried Matrix Spotting (DMS) cards. These compounds, being acidic in nature, are challenging in terms of achieving lower detection limits, except for atorvastatin. Poroshell 120 is an excellent column choice for running the analysis, because it offers speed and high resolution advantages comparable to sub-2 µm columns, but is more forgiving for dirtier samples due to its standard 2 µm frits.
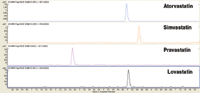
Figure 1: LCâMSâMS chromatogram of 50 ng/mL blood spiked with statins after DMS work-up.
DMS procedure
Fresh human whole blood (990 µL) was spiked with 10 µL/each of four statins, at 100× concentrated working standard to create a calibration curve of 20, 50, 100, 200, 500 and 2000 ng/mL. After vortexing, 15 µL of each concentration of spiked blood was spotted on Agilent Bond Elut DMS cards (Part #: A400150), which are non-cellulose in nature. For accuracy and precision, three replicates of blood concentrations at 20 ng/mL and 500 ng/mL were also prepared. Accuracy and precision studies were also extended to competitive cellulose-based cards. Cards, once spotted, were left overnight for drying. 3 mm disks were punched and placed in 2 mL vials. Each spot was dissolved in 300 µL desorption solvent (60% methanol with 1% ammonium hydroxide containing 0.5 ng/mL naproxen as an internal standard), and vortexed. Spots were left to soak in desorption solvent for ~2 hours, samples were then removed and put in conical vials, followed by evaporation to dryness. Samples were reconstituted in 100 µL of mobile phase (70% 5 mM Ammonium formate: 30% CH3CN) and vortexed.
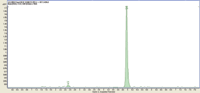
Figure 2: LCâMSâMS chromatogram of atorvastatin at1 ng/mL spiked blood.
Results and Discussion
Figure 1 is an example of 50 ng/mL spiked blood after work-up with DMS cards. The superficially porous particle technology of the Poroshell 120 column is designed to generate high efficiency separations at lower back-pressures. Back-pressures of 394 bar for this separation on a 150 × 2.1 mm column format on an ultra high pressure system such as an Agilent 1290 Infinity LC are impressive. Poroshell 120 EC-C8, an endcapped bonded phase, delivers excellent peak shapes for all analytes and is less retentive for non-polar analytes (all in the current mix except pravastatin). Amongst all the compounds examined, atorvastatin was the most sensitive. It could be detected easily at 1 ng/mL with a SNR of 797 (Figure 2), while others oculd barely be seen at 20 ng/mL.

Figure 3: Representative calibration curves of statins in spiked blood.
LC–MS conditions
Column: Poroshell 120 EC-C8, 2.7 µm, 150 × 2.1 mm (Part #: 693775-906)
Mobile phase: A: 5 mM Ammonium formate, B: CH3CN
Flow-rate: 200 µL/min
Gradient: t0 A: 70%, B: 30%
t5.0 A: 25%, B: 75%
t5.5 A: 25%, B: 75%
t5.6 A: 70%, B: 30%
t8.0 A: 70%, B: 30%
Column temp: 30 °C
Back pressure: 394 bar
Run time: 8 min
Instrument: Agilent 1290 Infinity LC / 6460 QQQ
Gas temp: 275 °C
Gas flow: 10 L/min
Nebulizer: 10 psi
Sheath gas temp: 250 °C
Sheath gas flow: 7 L/min
Polarity: Negative
Linearity was observed in the calibration curves for six levels for pravastatin and atorvastatin using linear regression with correlation coefficients better than 0.998 (Figure 3). Lovastatin and simvastatin curves were non-linear at the highest concentration and yielded quadratic regression with correlation coefficients better than 0.999.
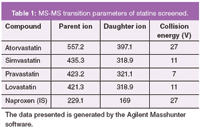
Table 1: MS-MS transition parameters of statins screened.
Table 3 lists relative recoveries of statins from blood after DMS desorption from Bond Elut DMS cards and competitive cards. The results indicate that there is ion-enhancement occurring with blood constituents when the cellulose product is used, resulting in artificially high recoveries. This data supports that the non-cellulose product exhibits better quality data for desorption compared to traditional cellulose cards.
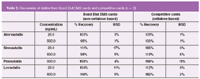
Table 2: Recoveries of statins from Bond Elut DMS cards and competitive cards (n = 3).
Conclusions
A simple and rapid method has been developed for the analysis of an acidic group of compounds in human DMS samples by LC–MS–MS. The method was demonstrated to be accurate, precise and robust. Linearity was demonstrated for pravastatin and atorvastatin using a linear regression with correlation coefficients better than 0.998 and good recoveries were seen for all analytes except pravastatin. These cards offered better desorption properties compared to the cellulose-based competitive product. A Poroshell 120 EC-C8, an excellent column choice for bioanalytical applications, yielded good peak shapes and enabled fast analysis with decreased back pressures. Dried blood spots should be considered as a sample collection technique when developing and validating quantitative bioanalytical methods for the analysis of drugs in pre-clinical and clinical studies.

Agilent Technologies Inc.
2850 Centerville Road, Wilmington, Delaware, USA
Website: www.agilent.com/chem/dmsappnote

Separating Impurities from Oligonucleotides Using Supercritical Fluid Chromatography
February 21st 2025Supercritical fluid chromatography (SFC) has been optimized for the analysis of 5-, 10-, 15-, and 18-mer oligonucleotides (ONs) and evaluated for its effectiveness in separating impurities from ONs.


















