Light Scattering for the Masses Kinetics of Enzyme-Inhibitor Associations
Wyatt Application Note
The kinetics of association or dissociation is of paramount importance in many biological systems. For example, antithrombin III forms a covalent bond with the catalytic serine of several proteases involved in the coagulation cascade, and the rate of this reaction can increase up to 1000× in the presence of certain macromolecules. Here, we determine the second-order rate constant for the covalent association of thrombin α (Thr) and antithrombin III (AT) by Composition Gradient Multi-Angle Light Scattering (CG-MALS).
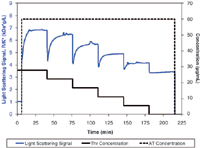
Figure 1: Light scattering and concentration data for thrombin titration at constant antithrombin concentration.
Human thrombin α and antithrombin III (Haematologic Technologies, Inc.) were diluted to 60 µg/mL and 120 µg/mL, respectively, in phosphate buffered saline (PBS, pH 7.4) and filtered to 0.02 µm. Composition gradients were created using a Calypso II and delivered to an online UV/vis concentration detector and DAWN HELEOS. The method consisted of six injections at constant AT concentration of 60 µg/mL and Thr concentrations from 0 to 30 µg/mL. After each injection into the UV and MALS detectors, the flow was stopped for 2000 s to allow the reaction to come to completion. Calypso software was used to run the method, acquire MALS and UV signals, and calculate the apparent weight-averaged molar mass as a function of time
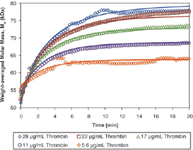
Figure 2: The increase in Mw as a function of time was fit to the appropriate association model to calculate the second order rate constant for the inhibition of Thr by AT, kon = 6.09 Ã103 Mâ1 sâ1. Raw data (open symbols) and best fit curves are shown for varying Thr concentrations and constant AT concentration of 60 µg/mL.
The light scattering data for all five measurements with nonzero thrombin concentrations were fit simultaneously to a model of 1:1 irreversible association between thrombin and antithrombin (Figure 2). Using this model, the second order rate constant was calculated as kon = 6.09 × 103 M–1 s–1 , similar to results measured by other methods (e.g., ka = 5.8 × 103 M–1 s–1 measured at 25 °C by fluorescence) (1). The analysis simultaneously determined the fraction of thrombin capable of binding antithrombin. This calculated fraction, 77%, compared favourably with the manufacturer's reported specific activity of the antithrombin (0.79 mol Thr/mol AT).
Thus, CG-MALS provided a complete picture of the association between thrombin and antithrombin in solution, including identifying a fraction of thrombin incapable of binding its inhibitor. This technique enabled observation of slow, irreversible binding and measurement of rates of 1:1 association consistent with other techniques.
References
1. G. Izaguirre et al., Proc. Natl. Acad. Sci., 282, 33609–33622 (2007).
DAWN®, miniDAWN®, ASTRA®, Optilab® and the Wyatt Technology logo are registered trademarks of Wyatt Technology Corporation.

Wyatt Technology Corp.
6300 Hollister Ave., Santa Barbara, California, USA
tel: +1 805 681 9009 fax: +1 805 681 0123
E-mail: info@wyatt.com Website: www.wyatt.com

Extracting Estrogenic Hormones Using Rotating Disk and Modified Clays
April 14th 2025University of Caldas and University of Chile researchers extracted estrogenic hormones from wastewater samples using rotating disk sorption extraction. After extraction, the concentrated analytes were measured using liquid chromatography coupled with photodiode array detection (HPLC-PDA).
Silvia Radenkovic on Building Connections in the Scientific Community
April 11th 2025In the second part of our conversation with Silvia Radenkovic, she shares insights into her involvement in scientific organizations and offers advice for young scientists looking to engage more in scientific organizations.
Regulatory Deadlines and Supply Chain Challenges Take Center Stage in Nitrosamine Discussion
April 10th 2025During an LCGC International peer exchange, Aloka Srinivasan, Mayank Bhanti, and Amber Burch discussed the regulatory deadlines and supply chain challenges that come with nitrosamine analysis.


















