Determination of Explosives Using Fast and High Resolution Liquid Chromatography with the Agilent 1290 Infinity LC System
The Application Notebook
Agilent Application Note
This application note describes the method for detection of nanogram levels of explosive constituents in seawater samples (e.g., after detonation of unexploded ordnance devices). The improved chromatographic method was developed using the Agilent 1290 Infinity LC Method Development system.
Long after World War II, unexploded ordnance devices are sometimes found, not only in the ground but also near the coasts, such as in the Baltic Sea. These old munitions are often corroded and cannot be deactivated; sometimes, the only way to deactivate is with a controlled detonation. The residues of the detonation that can be found in the water must be monitored because of their toxicity. This application note describes the method for detection of nanogram levels of explosive constituents in seawater samples. The improved chromatographic method was developed using the Agilent 1290 Infinity LC Method Development system.
Experimental
LC System
For method development and analysis of the samples, an Agilent 1290 Infinity LC system was used. The system consists of: 1290 Infinity Binary pump with integrated vacuum degasser, 1290 Infinity high performance autosampler, 1290 Infinity Thermostatted column compartment and 1290 Infinity Diode Array Detector with 10- and 60-mm Max Light Cartridge flow cell.
Analytes
Components: Octogen (HMX), Hexogen (RDX), 2,4-Dinitrotoluol (2,4-DNT), 2,6-Dinitrotoluol (2,6-DNT), 2,4,6-Trinitrotoluol (TNT), Nitropenta (PETN), Nitroglycerine, 2,4,6-Trinitrophenylmethylnitramine (Tetryl), 2,3-Dinitrodimethylbutane (2,3-DNDMB-internal standard, eluting at 3.35 min in Figure 1).
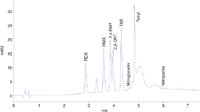
Figure 1: Detection of the 5 μg/mL standard with the 60-mm high sensitivity cell at 235 nm. Chromatographic conditions: Stationary phase: Stable Bond CN-RRHT (2.1 à 100 mm, 1.8 μm); Mobile phase A: Water; Mobile phase B: Acetonitrile; Gradient: at 0 min 20 % B, at 6 min 40 % B, at 7 min 40 % B; Flow-rate: 0.5 mL/min; Column temp: 30 °C; Inj. vol: 1 μL; UV Detection: 214 nm/4, Ref: 450 nm/80; Data rate: 20 Hz.
Results and Discussion
The explosives analysed in this application are very polar but not water-soluble substances, and are not well separated on typical RP18 columns. Therefore, different RP columns, including more polar materials, were systematically screened using the Agilent 1290 Infinity Method Development system. Great selectivity differences can be seen between the unpolar phases like Eclipse Plus C18, Eclipse Plus Phenyl-Hexyl, Poroshell EC-C18 and Stable Bond C18. Only the Phenyl-Hexyl column shows separation of more than four of the explosives. The polar RP-phases are more useful. In particular, the critical pair 2,4- and 2,6-dinitrotoluol is only completely separated on the Bonus RP and the SB-AQ, but with the disadvantage of coelution of TNT and Tetryl. The best separation with a simple gradient was achieved with the Stable Bond CN column, and therefore, this phase was used for further optimization. The final method separates all explosives within 7 min. A further improvement in sensitivity was achieved by using the new 60-mm high sensitivity flow cell. Figure 1 shows the chromatograms of the 5 μg/mL standard with the 10 mm cell (data not shown) and the 60 mm high sensitivity cell. If the detection wavelength is changed from 235 nm to 214 nm, the influence of the eluent might be increased. However, the sensitivity for 2,3-DNDMB, nitropenta and nitroglycerine is further increased.
Conclusion
The new Agilent 1290 Infinity LC is designed to provide the highest speed, resolution and sensitivity. The transfer of a standard method to determine eight explosives on sub-2-μm RRHD or RRHT columns could be done "overnight" by identifying the best selectivity using the Agilent Method Development system. The results show that the analysis could be shortened to 7 min without any matrix influences.

Agilent Technologies Inc.
5301 Stevens Creek Blvd., Santa Clara, California 95051, USA
tel: +1 877 424 4536 fax: +1 408 345 8474
Website: www.agilent.com

New Method Explored for the Detection of CECs in Crops Irrigated with Contaminated Water
April 30th 2025This new study presents a validated QuEChERS–LC-MS/MS method for detecting eight persistent, mobile, and toxic substances in escarole, tomatoes, and tomato leaves irrigated with contaminated water.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)

















