Increased Sensitivity, Improved Resolution and Faster Analysis Times For Compendial Methods: Determination of Impurities and Related Substances Using Newly Developed 2.6 µm Core-Shell Kinetex LC Columns
Recently a newly developed Kinetex 2.6 µm Core-Shell chromatographic particle has been commercialized that offers the performance benefits of sub-2 µm fully-porous particles but at substantially lower operating pressures.
Recently a newly developed Kinetex™ 2.6 μm Core-Shell chromatographic particle has been commercialized that offers the performance benefits of sub-2 μm fully-porous particles but at substantially lower operating pressures. To demonstrate the performance benefits of this new core-shell technology, a Kinetex™ 2.6 μm Core-Shell C18 column was compared with a fully-porous 5 μm C18 column referenced in EP ([Ph. Eur.] European Pharmacopoeia) Monograph 0703 for Atenolol and related substances on a conventional HPLC instrument with an upper pressure limit of 400 bar.
Atenolol and Related Substances: European Pharmacopoeia Monograph 0703
Experimental
Columns Used: A fully-porous 5 μm C18 125 × 4.0 mm column (as specified by the monograph) was compared with a Kinetex™ 2.6 μm C18 100 × 4.6 mm column (the closest available dimension).
Instrumentation: Agilent 1100 LC System (Agilent Technologies Inc., Palo Alto, CA, USA) equipped with a Quaternary gradient pump, autosampler, column oven, and variable wavelength detector.
Sample Preparation and Analysis: Atenolol Certified Reference Standard (CRS) for system suitability (containing atenolol and impurities B, F, G, I, and J) was obtained from the European Pharmacopoeia. 5 mg of Atenolol CRS was dissolved in 2.5 mL of the mobile phase. The monograph calls for isocratic elution with 100 % of mobile phase (as specified in the monograph) at 0.6 mL/min. Column temperature kept at ambient and UV detection wavelength set at 226 nm.
Results
Following the methodology described in European Pharmacopoeia Monograph 0703 and using a fully-porous 5 μm C18 125 × 4.0 mm column as referenced in the method, a chromatogram similar to that of the specimen chromatogram provided with the Atenolol CRS was obtained (Figure 1).
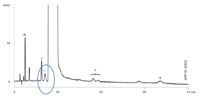
Figure 1: Atenolol CRS: Fully-porous 5 μm C18 125 à 4.0 mm at 10 μL injection 0.6 mL/min.
A Kinetex™ 2.6 μm C18 100 × 4.6 mm column (the closest available dimension) was used according to the conditions specified in the monograph. The resulting chromatogram demonstrated equivalency for selectivity and also demonstrated significantly improved sensitivity (Figure 2).
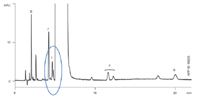
Figure 2: Atenolol CRS: Kinetex 2.6 μm C18 100 à 4.6 mm at injection 0.6 ml/min.
Table I summarizes the data comparing the Kinetex™ column to the fully-porous 5 μm column at the specified flow rate of 0.6 mL/min. The monograph requires resolution between impurities I and J of at least 1.4. Due to the significantly narrower peaks generated by the higher efficiency Kinetex™ column, a substantial improvement in resolution between impurities I and J was achieved with Kinetex™.
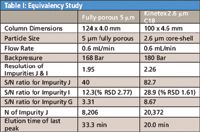
Table I: Equivalency Study
Sensitivity was also significantly improved for all impurities as a result of the Kinetex™ column generating narrower and taller peaks. The higher signal-to-noise ratios and subsequently lower %RSD values observed with the Kinetex™ core-shell technology represent a significant performance advantage. It should be noted that the Kinetex™ column generated a system pressure comparable to the 5 μm column under these conditions.
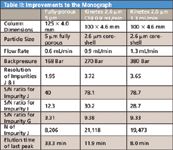
Table II: Improvements to the Monograph
Significantly Faster Analysis Times with Kinetex™
Due to favorable physical, kinetic, and thermodynamic properties attributed to core-shell particles. Shorter analysis times may be achieved with Kinetex™ by reducing the length of the column or increasing the mobile phase flow rate (or a combination of both) without significantly compromising chromatographic performance.
Staying within the European Pharmacopoeia guidelines outlining the extent to which parameters of a chromatographic test may be adjusted to satisfy system suitability (when replacing one column with another of the same type, for example) the Kinetex™ 2.6 μm core-shell C18 column was run with a 50% increase in the flow rate (from 0.6 mL/min to 0.9 mL/min). Total analysis time was shortened from over 33 min to just under 12 min (Figure 3).
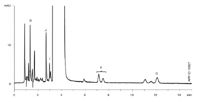
Figure 3: Atenolol CRS: Kinetex 2.6 μm C18 100 à 4.6 mm at injection 0.9 ml/min.
Conclusions
Laboratories performing routine API and related substance analysis with traditional fully-porous LC columns can benefit from the increased speed, resolution, and sensitivity that Kinetex™ 2.6 μm core-shell columns provide without having to replace existing instrumentation with ultra-high pressure capable LC systems. Faster analysis times resulting in higher throughput and productivity can be achieved with Kinetex™ columns with minimal changes to validated methods by employing shorter length columns and/or higher mobile phase flow rates without sacrificing performance. Improved resolution and higher sensitivity resulting from narrower and taller chromatographic peaks generated by Kinetex™ columns allow for more precise detection and quantitation of low level impurities in routine operation.

Phenomenex, Inc.
411 Madrid Ave, Torrance, CA 90501
tel. (310)212-0555, (310)328-7768
Website: www.phenomenex.com

SEC-MALS of Antibody Therapeutics—A Robust Method for In-Depth Sample Characterization
June 1st 2022Monoclonal antibodies (mAbs) are effective therapeutics for cancers, auto-immune diseases, viral infections, and other diseases. Recent developments in antibody therapeutics aim to add more specific binding regions (bi- and multi-specificity) to increase their effectiveness and/or to downsize the molecule to the specific binding regions (for example, scFv or Fab fragment) to achieve better penetration of the tissue. As the molecule gets more complex, the possible high and low molecular weight (H/LMW) impurities become more complex, too. In order to accurately analyze the various species, more advanced detection than ultraviolet (UV) is required to characterize a mAb sample.















