Improved Clean Up and Recovery of Pharmaceutical Compounds From Plasma Using Strata-X Solid Phase Extraction vs Traditional Liquid-Liquid Extraction
Phenomenex Application Note
Diclofenac is a slightly acidic non-steroidal anti-inflammatory drug (NSAID) that has been used as a post operative pain reliever in adult and pediatric patients. The subsequent quantification of diclofenac from the small volumes of biological matrices, such as plasma, has been of significant concern. Therefore, this article explores two popular extraction methods, solid phase extraction (SPE) and liquid-liquid extraction (LLE), for the isolation of diclofenac from plasma, using a water matrix as the control.
Materials and Methods
The plasma pre-treatment step was the same for SPE and LLE and was comprised of filtration through a gauze cloth. Afterwards, 500 µL of diclofenac, which was dissolved in 5% Methanol, was added to 500 µL of plasma, and the solution mixture was then acidified with 600 µL of 1 M Phosphoric acid.
Solid Phase Extraction
The pre-treated plasma samples were further cleaned up and concentrated using SPE as shown in Figure 1.

Figure 1: SPE.
Liquid-Liquid Extraction
After pre-treatment, 5 mL of hexane:IPA (95:5) was added to the pre-treated solution, which was followed by 1 minute of vortexing, and 10 minutes of centrifugation at 2000 rpm. Subsequently, 4 mL of the top organic layer was transferred to a clean glass centrifuge tube and then evaporated to dryness under a stream of nitrogen at 53 °C for 20 minutes.
LC/UV
Figure 2 shows a diclofenac spiked plasma sample (50 µg/mL) after extraction with Strata-X. Flurbiprofen (IS) was added post-extraction (after blow-down) at a concentration of 160 µg/mL.
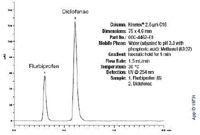
Figure 2: LC/UV.
Results and Discussion
Traditionally, liquid-liquid extraction (LLE) has been used as the standard clean up procedure for a variety of pharmaceutical samples. While performing this work, it was discovered that solid phase extraction provided many benefits over LLE including improved recoveries, time and solvent savings, as well as more consistent results (Table 1).
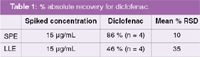
Table 1: % absolute recovery for diclofenac.
Conclusion
The Strata-X SPE sorbent uses the chemical bonding properties of a pyrrolidone ligand, which makes it a prime target for the retention of hydrophobic neutral compounds, while also retaining basic and acidic compounds under strong organic wash conditions.
LLE instead used two immiscible solvents that compete for interaction with the analyte of interest. While LLE has been a universal choice of extraction, SPE poses many advantages over LLE for the extraction of diclofenac. Consequently, this data shows that SPE provides greater absolute recovery of diclofenac when compared to LLE; and is less time-intensive, consumes less solvent than traditional LLE procedures, and provides better reproducibility, thereby demonstrating that the extraction method of choice for pharmaceuticals, such as diclofenac, is SPE.
Phenomenex Inc.
411 Madrid Ave., Torrance, California, USA
tel: +1 310 212 055 fax: +1 310 212 7768
Website: www.phenomenex.com

Separating Impurities from Oligonucleotides Using Supercritical Fluid Chromatography
February 21st 2025Supercritical fluid chromatography (SFC) has been optimized for the analysis of 5-, 10-, 15-, and 18-mer oligonucleotides (ONs) and evaluated for its effectiveness in separating impurities from ONs.



















