Steviol Glycosides: Fast On-line SPE for Sample Cleaning of Plant Extracts
The Application Notebook
KNAUER Application Note
Introduction
Sample cleanup via solid phase extraction (SPE) and similar methods are time-consuming and cost-intensive procedures. In particular, the purification of complex plant extracts requires special care. Here we introduce a robust and sensitive on-line SPE sample preparation method which was used for the determination of steviol glycosides. Starting with a filtered plant extract, the purification step and steviol glycoside determination requires less than 13 minutes.
Instrumentation
The SPE purification and the following determination were performed on a KNAUER PLATINblue HPLC Plus system with two P-1 Pumps (one for analysing and one for transport), Autosampler AS-1, Column Thermostat T-1 with two integrated 6/1 port 1/32" valves, Detector PDA-1, Assistant with two 1/8" solvent selection valves and one separate two way 1/16" valve.
Experimental
Extraction (Manual): Step 1: Extraction with acetonitrile/water 70:30 (v/v). Step 2: Filtration of the extract through 0.45 µm filter.
Extract purification (Cleanup): Step 1: Injection of 10 µL extract. Step 2: Sample transport to SteviaClean cartridge: cleaning step with acetonitrile/water 90:10 (v/v), 2 min at a flow-rate of 2 mLmin.
Sample Analysis: Switching the SteviaClean cartridge into the determination part of the HPLC system for 30 seconds and starting the PDA acquisition immediately after switching.
HPLC Method: Column: Eurospher 100-5 NH2, 150 × 3 mm. Mobile phase: acetonitrile/water 80:20 (v/v). Flow-rate: 1.0 mL/min. Temperature: 35 °C. Detection: UV, 210 nm.
SteviaClean Cartridge Cleaning and Conditioning (During Sample Analysis): Step 1: After switching back, flushing with water for 4 min at a flow-rate of 3 mL/min. Step 2: Flushing 4 min with acetonitrile/water 90:10 (v/v) at a flow-rate of 3 mL/min and then at 0.5 mL/min flow until the method ends.
Results
As shown in Figure 1 the on-line SPE purification works very fast and robustly with the KNAUER SteviaClean cartridge. A complete purification and determination run can be accomplished within 13 minutes. For standard impurity level plant extracts the SteviaClean cartridge can be used up to 10 times without any penetration of matrix components or losses of stevioside and rebaudioside A. During the subsequent determination, the SteviaClean cartridge was flushed with the listed mobile phases. The slightly shifted retention times between the two runs result from the injection of the purified sample directly from the cartridge. For ten measurements the relative standard deviation of the peak area was 0.4% for stevioside and 0.9% for rebaudioside A. The recovery rate was 107% for stevioside and 99% for rebaudioside A.
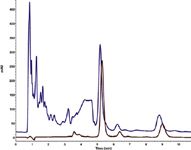
Figure 1: Comparison of non-purified extract (blue) and purified extract (red).
Conclusion
This on-line SPE purification method applying KNAUER SteviaClean SPE cartridges in combination with a KNAUER PLATINblue HPLC Plus system is able to reliably purify and determine a stevia plant extract in less than 13 minutes. With the two 6/1 port 1/32" valves installed in the KNAUER Column Thermostat T-1, it is possible to install up to six KNAUER SteviaClean cartridges for the determination of up to 60 samples automatically with an overall run time of approximately 15 hours. Therefore it is a very suitable method for high throughput analysis.
References
1. Steviol Glycosides, Prepared at the 73rd JECFA (2010), published in FAO JECFA Monographs, 10 (2010).
Wissenschaftliche Gerätebau Dr. Ing. Herbert Knauer GmbH
Hegauer Weg 38, 14163 Berlin, Germany
tel: +49 30 809727 0 fax: +49 30 801501 0
E-mail: info@knauer.net Website: www.knauer.net

HPLC 2025 Preview: Fundamentally Speaking (Part 1)
May 13th 2025Michael Lämmerhofer from the Institute of Pharmaceutical Sciences, University of Tübingen, Germany, spoke to JFK Huber Lecture Award winner of 2024 Torgny Fornstedt, professor in analytical chemistry and leader of the Fundamental Separation Science Group, Karlstad University, Sweden, about his pioneering work in high performance liquid chromatography (HPLC) with a focus on fundamentals and industrial applications.
Reversed-Phases for LC Deliberately Doped with Positive Charge: Tips and Tricks for Effective Use
May 13th 2025In this month's edition of LC Troubleshooting, Dwight Stoll and his fellow researchers discuss both the benefits (improved peak shape/loading) and challenges (excessive interaction) associated with charge-doped reversed-phase (RP) columns for both analytical and preparative separations.
Determining Ways to Protect Honeybee Colonies with GC–MS
May 13th 2025A study conducted by the Agriculture Research Centre of Giza, Egypt, and Jilin Agricultural University in China, evaluated the efficacy of stinging nettle extract, nettle smoke, and formic acid in the controlling of Varroa mites, a major threat to honeybee colonies, with a focus on mite infestation reduction, honeybee mortality, and biochemical responses. Gas chromatography–mass spectrometry (GC–MS) was used to identify key bioactive compounds in the stinging nettle extract.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)



















