Hyphenated Techniques as Modern Detection Systems in Ion Chromatography
Metrohm Application Note
Andrea Wille, Stefanie Czyborra and Alfred Steinbach, Metrohm International Headquarters, Herisau, Switzerland.
This article describes the use of combined ion chromatography-mass spectrometry (IC–MS) and ion chromatography-inductively coupled plasma mass spectrometry (IC-ICP–MS) to analyse potentially harmful compounds.
Introduction
Ion chromatography (IC) with conductivity detection has been successfully used to analyse low-molecular organic compounds such as amines and organic acids, as well as anionic and cationic substances. However, because of current toxicity concerns and the consequently lowered maximum contaminant levels (MCL), analyses in complex matrices require improved sensitivity and selectivity.
The coupling of IC with multidimensional detectors such as an electrospray ionization mass spectrometer (ESI-MS) or an inductively coupled plasma mass spectrometer (ICP–MS) solves even complex separation problems, simultaneously achieving outstanding sensitivity and selectivity. Additionally, these so-called hyphenated techniques provide valuable information for an unambiguous peak identification and are less prone to matrix influences than conductivity detection. While IC-ICP–MS is used for element-specific analysis, IC–MS allows the identification of parent compounds and corresponding metabolites.
This article deals with the determination of bromate, perchlorate and organic acids by IC–MS as well as the detection of inorganic and organic chromium, arsenic and selenium species by IC-ICP–MS.
Experimental
Instrumentation
The analytical system used consisted of the 858 Professional sample processor and the 850 Professional IC Anion – MCS (Metrohm AG, Switzerland). The detectors were the 1100 MSD SL (MS) and the 7500 ICP–MS (both Agilent Technologies, USA). The separation columns and instrumental parameters used for IC–MS and IC-ICP–MS are listed in Tables 1 and 2, respectively.

Table 1: IC conditions and MS operating parameters used for the analysis of bromate, perchlorate and the organic acids.
Standard solutions and eluents
All reagents used in this work were of the highest purity grade (puriss. p.a.). The oxyhalide, chromium, arsenic and selenium standard solutions were all purchased from Fluka (Sigma Aldrich, Buchs, Switzerland). All solutions were prepared with deionized water with a specific resistance higher than 18 MΩ·cm.

Table 2: IC conditions and ICPâMS operating conditions used for the analysis of chromium, arsenic and selenium.
Results and Discussion
IC–MS
The coupling of the separation power of an IC with the detection power of an MS has opened up entirely new possibilities. Adequate chromatographic retention of the target compounds is combined with the sensitive detection of MS, providing outstanding selectivity and sensitivity. Additionally, identification based on simultaneous retention time matching and isotope ratios reduces the risk of incorrect peak assignment (false positives).
Bromate in drinking water
Bromate, a potential carcinogen, is generated by the oxidation of bromide traces during water disinfection, for example by ozonization. For drinking water and mineral water, current regulations stipulate a limit of 10 and 3 μg/L (ppb), respectively. Other oxyhalides (ClO3–, ClO2–) and standard anions (Br–, NO3–, H2PO4–, HSO4–) can be determined in the same run (Figure 1).1
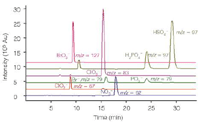
Figure 1: MS chromatogram of a 10 mg/L standard for the standard anions and oxyhalides.
Perchlorate in drinking water
Perchlorate is an environmentally stable, highly water soluble contaminant that is commonly used as an oxidant in solid fuel rocket propellants. It has been shown to competitively inhibit the transport of iodine into the thyroid gland. Recently, the Environmental Protection Agency (EPA) developed an IC-ESI-MS method (EPA 332) for drinking water with a detection limit in the sub-μg/L range. Figure 2 displays two overlaid chromatograms, one of a slightly contaminated groundwater sample and one of the same sample spiked with 1 μg/L of perchlorate.2,3
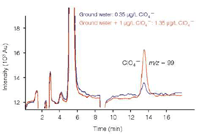
Figure 2: MS chromatogram of an unchanged groundwater sample and a groundwater sample spiked with 1 μg/L perchlorate. Because of the higher isotopic abundance of the 37Cl isotope, only the SIM trace m/z 99 is displayed.3
Organic acids in produced water
Produced water is water trapped in underground reservoir rocks that is brought to the surface along with the crude oil and gas. Besides dispersed oil droplets and significant amounts of inorganic ions, produced water contains dissolved organic acids, mainly stemming from the degradation of organic matter. Generally, these acids point to the presence of oil.
The analytical challenge consists in detecting sub-mg/L quantities of carboxylates in the presence of very high concentrations of sodium and chloride. As Figure 3 demonstrates, IC–MS coupling allows reliable quantification of carboxylic acids, such as acetate, propionate and butyrate in the presence of a high salt matrix containing approximately 100 g/L chloride.
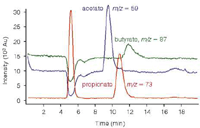
Figure 3: MS Chromatogram of a produced water sample containing 428.4 mg/L acetate, 9.3 mg/L propionate and 2.6 mg/L butyrate.
IC-ICP–MS
The 'zero tolerance' policy concerning chromium, arsenic and selenium compounds in drinking water has led to IC-ICP–MS becoming one of the most prominent detection methods for trace metals in environmental samples. The advantage of this technique is that it can distinguish between different oxidation states and chemical forms of a particular element. This approach is called speciation analysis. Individual concentrations of element-containing species are, from the toxicological point of view, far more significant than total element concentrations as different valence states of an element have different properties. Chromium, arsenic and selenium species can either be identified by their retention times or isotopic ratios.
Chromium
Chromium compounds are used in dye and pigment production, in cement, in tanning, as mordants, wood preservatives and as efficient corrosion inhibitors. While chromium(III) is an essential trace element for humans, all forms of hexavalent chromium are regarded as highly toxic and carcinogenic. Accordingly, the EPA has fixed the MCL of chromium(VI) at 100 μg/L. Figure 4 shows the chromatogram of a 0.1 μg/L standard with the trivalent and hexavalent chromium peaks.4
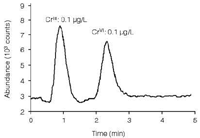
Figure 4: Separation and detection of chromium(III) and (VI), each at a concentration of 0.1 μg/L.5
Arsenic
Arsenic is the twentieth most abundant element in the earth crust, ubiquitously found in a high number of minerals. It is used in pesticides, wood preservatives, semiconductors, etc. Its use as a weed killer and rat poison illustrates its high toxicity. Additionally, inorganic arsenical derivatives are considered to be carcinogenic and maybe teratogenic. Therefore, the EPA proposes a maximum allowable drinking water concentration of 10 μg/L.
In environmental and biological samples, more than 20 arsenic species have been identified. Depending on their binding characteristics, they have different toxicities and chemical behaviour. IC-ICP–MS allows the separation and unambiguous identification, based on structural data, of different arsenic species in inorganic and organic forms (Figure 5).
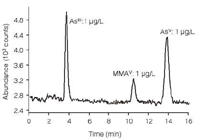
Figure 5: Separation and detection of arsenious acid (AsIII), monomethylarsonic acid (MMAV) and arsenic acid (AsV), each at a concentration of 1 μg/L.
Selenium
Because selenium compounds also differ strongly in their toxicity, speciation analysis is of paramount importance (Figure 6). Selenium is used in food supplements, photographic processes and electronics. Selenium is an essential trace element for humans and animals. Its uptake occurs mainly through food (grains, cereals and meat) and water. Elevated concentrations, however, are considered to adversely affect health (MCL = 50 μg/L). An increasing number of biogeochemical research papers point to the fact that selenium compounds have severe impacts on aquatic wildlife.5
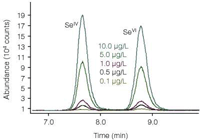
Figure 6: Separation and detection of Selenite (SeIV) and Selenate (SeVI) at concentrations between 0.1 and 10 μg/L.
Conclusions
The coupling of highly efficient ion chromatography (IC) to multidimensional detectors such as a mass spectrometer (MS) or an inductively coupled plasma mass spectrometer (ICP–MS) significantly increases sensitivity while simultaneously reducing matrix interference. IC–MS can detect several oxyhalides, such as bromate and perchlorate, in the sub-ppb range. Additionally, organic acids can be precisely quantified through mass-based determination even in the presence of high salt matrices.
By means of IC-ICP–MS, different valence states of chromium, arsenic and selenium in the form of inorganic and organic species can be sensitively (0.01–0.1 μg/L) and unambiguously identified in one single run. Moreover, IC-ICP–MS is a valuable tool for the robust and sensitive analysis of transition metals.
References
1. G. Bogenschütz et al., Advanced detection techniques in ion chromatography, Metrohm Monograph, Herisau (2007) in press.
2. A. Wille and S. Czyborra, IC–MS coupling — theory, concepts and applications, Technical Paper, Metrohm AG, Herisau (2007).
3. J. Mathew, J. Gandhi and J. Hedrick, The analysis of perchlorate by ion chromatography/mass spectrometry, Agilent Application (2004).
4. T. Sakai, E. McCurdy and S. Wilbur, Ion chromatography (IC) ICP–MS for chromium speciation in natural samples, Agilent Application (2005).
5. A.D. Lemly, Exotoxicology and environmental safety, 59, 44–56 (2004).

Metrohm International Headquarters
Oberdorfstrasse 68, CH-9101 Herisau, Switzerland
tel. +41 71 353 85 04
E-mail: aw@metrohm.com
Website: www.metrohm.com















