Novel Analytical Methods for the Discovery and Trace Analysis of Biochemically Active Compounds
LCGC Asia Pacific
Novel analytical methods for the discovery and trace analysis of biochemically active compunds in three main area are described: protein analysis, screening technologies and multidimensional separations.
Jeroen Kool, Henk Lingeman, Wilfried Niessen and Hubertus Irth, BioMolecular Analysis group, Faculty of Science, Amsterdam, The Netherlands.
This article will discusss novel analytical methods for the discovery and trace analysis of biochemically active compunds in three main areas: protein analysis, screening technologies and multidimensional separations. The research in protein analysis describes analytical methods that involve automation and integration of sample clean-up, followed by separation and detection of the peptides/proteins.
On-line digestion procedures before — or after — sample clean-up to allow tandem mass spectrometry (MS–MS)-based peptide fingerprinting or sequence analysis for identification purposes is useful for the analysis of biomacromolecules, such as proteins. This allows the analysis of proteins with large similarities or proteins that are covalently modified as a result of reactive chemicals or post-translational modifications.
In brief
Hyphenated techniques applying enzymatic or immunological immobilization techniques are currently important topics. Methods using on-line digestion with immobilized or solution-phase enzyme reactors (using trypsin, pepsin and other proteases) and subsequent liquid chromatography mass spectrometry (LC–MS) analysis of marker peptides have been developed. Recent applications of this methodology include the determination of covalent adducts of warfare agents to endogenous proteins as well as the trace analysis of human serum albumin (HSA) adducted to reactive intermediates of therapeutic drugs.
To highlight some of the recent advances in this area, three specific applications are discussed: (1) the development of an automated on-line pepsin digestion–LC–MS configuration for the rapid determination of protein adducts of chemical warfare agents. (2) the automated detection of covalent adducts to HSA by immunoaffinity chromatography and on-line solution phase digestion and LC–MS and (3) the trace analysis of proteins using post separation solution-phase digestion and MS detection of marker peptides.
Development of an automated on-line pepsin digestion–LC–MS configuration for the rapid determination of protein adducts of chemical warfare agents
Rapid monitoring and retrospective verification are crucial to protect against and aid the non-proliferation of chemical warfare agents. Monitoring and verification is currently performed by LC–MS to determine persistent protein adducts of these agents. Unfortunately, this procedure is rather elaborate. Therefore, an automated on-line pepsin digestion-LC–MS method has been developed for the efficient and rapid determination of chemical warfare agents adducted to proteins (Figure 1).1 The automated system used an on-line pepsin digestion unit coupled to LC–MS.1 The system allows the injection of samples into a flow-through pepsin immobilized cartridge in which injected proteins are digested, followed by subsequent trapping of the peptides formed on a sequentially placed LC-column. A switching-valve now places the LC-column, containing the trapped peptides, into a gradient LC system connected to the MS for analyte detection.
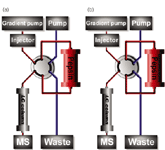
Figure 1: Schematic representation of the analysis system. The configuration consists of a switching valve connected to a gradient LC pump, a sampler (inject), a pepsin cartridge, an isocratic LC pump and an LC column directly coupled to the mass spectrometer. (a) Shows the valve connections during the injection of the sample, digestion, and trapping on the LC column. (b) Shows the valve after switching for gradient elution of the peptides from the LC column and concomitant reconditioning of the pepsin cartridge.
The applicability of the system was demonstrated for the detection of sulphur mustard and sarin exposure. Fast analysis times and less laborious sample-processing procedures allowed a high throughput of samples, which is advantageous for monitoring incidents with chemical agents. For a typical adducted protein, the peptide-mustard adduct (LQQC*PFEDHVKL) with the covalent modification on the cysteine 34 (C*) position could be detected after the on-line pepsin digest configuration in a straightforward manner (Figure 2). The site of modification was deduced from the product ion mass spectrum because the C-terminal fragments up to y"8 and N-terminal fragments up to b3 corresponded to unmodified amino acid residues. Furthermore, it was shown that residence times in the pepsin digestion unit for longer than 5 min allowed the same level of adduct peptides detected, thus demonstrating that this incubation time is satisfactory for the current system. It was shown for an adduct nonapeptide that a yield of 70% was obtained compared with traditional off-line in-solution digestion. Similar digestion yields were also obtained for other non-adducted proteins.
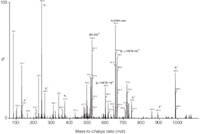
Figure 2: Product ion mass spectrum of [M + 3H]3+, m/z = 520.9, of LQQC*PFEDHVKL from a pepsin digest of human serum albumin exposed to sulphur mustard (HETE = 2-hydroxyethylthioethyl). m/z = 651.8 results from H2O/NH3 loss from parent ion.
Automated detection of covalent adducts to HSA by immunoaffinity chromatography, on-line solution phase digestion and LC–MS
In a similar methodology, but using a slightly different set-up, the detection of covalent adducts to the cysteine-34 residue of HSA was achieved.2 This particular set-up is based on the on-line combination of immunoaffinity chromatography, solution-phase digestion, LC and MS–MS. Immobilized anti-HSA antibodies are used for sample pre-concentration and purification of HSA and modified (e.g., alkylated) HSA followed by elution into an on-line digestion chamber with infused pronase. Subsequently, digestion products are trapped on-line on a C18 SPE cartridge followed by an LC separation with MS–MS detection. HSA was covalently modified by model alkylating agents [e.g., the reactive metabolite of acetaminophen, N-acetyl-p–benzoquinoneimine (NAPQI) and/or the alkylating agent 1-chloro-2,4-dinitrobenzene (CDNB)]. The alkylation of HSA was clearly demonstrated by the detection of the modified marker tetra-peptide glutamine-cysteine-proline-phenylalanine (QCPF) adduct of NAPQI-QC*PF and CDNB-QC*PF. To distinguish between unmodified and modified HSA samples, data comparison algorithms were used. As the focus of the method was the detection of covalent adducts to the cysteine-34 residue of HSA, no attention was paid on the determination of sequence coverages. The in-solution digestion proved to be a useful tool for reproducible and fast (less than 2 min) determination of covalent adduct formation to HSA with detection limits of 1.5 µM for modified HSA with only 10 µL of sample. In a previous set-up developed for quantitative detection of target proteins in general and HSA specifically, an immobilized trypsin reactor was used instead of a solution-phase reactor.3 Although well suited for quantitative detection of HSA, this method suffered from a low but reproducible trypsin digestion process (ca. 4%).
Trace analysis of proteins using post separation solution-phase digestion and MS detection of marker peptides4
The potential of post-column on-line in-solution digestion coupled to LC–MS was also explored for absolute quantification of intact proteins in mixtures (e.g., therapeutic proteins and their metabolites). Different approaches for the quantification of proteins have recently been reviewed by Hopfgartner and Varesio.5 Using traditional methodologies, complicated analysis strategies such as ELISAs or off-line and on-line digestion methods implemented prior to LC–MS can be performed. Evidently, these strategies cannot be applied conveniently to mixtures of proteins with high sequence homologies. In such a case it is essential to separate proteins prior to analysis by, for example, digestion and quantitative MS. To this end, a solution-phase digestion methodology based on infusion of pepsin that was placed post-column to a nano-C18-LC column was developed.4 Specific peptides formed from the eluting target protein(s) were used for quantification. For five model proteins, marker peptides with m/z in the range of 300–1000 with detection limits from 30–90 fmol protein of the original protein could be quantified accurately with linear responses ranging up to 3 pmol. Although the focus was on target analysis of marker peptides, sequence coverages of bovine serum albumin and horse heart myoglobin were determined for evaluation purposes and found to be 13% and 72%, respectively.
Screening Technologies
For the discovery and trace analysis of biochemically active compounds in mixtures, two fundamentally different analytical approaches are explored by our group. The first approach is the on-line coupling of different biochemical assay formats after LC separations.6–12 This allows the direct assessment of bioactivity from eluting compounds with simultaneous identity profiling by parallel on-line MS analysis. This approach allows the rapid and sensitive elucidation of compounds with valuable biochemical activities. However, for delicate biochemical assay formats an approach has been developed in which pre-analytical incubations of complex mixtures using native biochemical conditions are followed by fully automated at-line trapping of the target protein with bound bioactive compounds (ligands). Subsequently, rapid on-line clean-up steps and LC–MS allow the identification of all bioactive compounds extracted. Applying this general approach, five distinct and novel specific analytical methodologies have been introduced: (1) Analysis of protein-ligand interactions using dynamic protein-affinity chromatography SPE–LC–MS, (2) development of a counter gradient parking system for gradient LC with on-line biochemical detection of serine protease inhibitors, (3) application of hyphenated post-column biochemical assays, high resolution MS and NMR for the characterization and identification of estrogenic metabolites formed by drug-metabolizing Cytochromes P450, (4) on-line assessment of catalyst performance and (5) analysis of targeted toxic small molecule biomarkers.
Analysis of protein–ligand interactions using dynamic protein-affinity chromatography solid-phase extraction–liquid chromatography–mass spectrometry13
The necessity for finding new lead compounds in drug discovery has strengthened the need for analytical methods that are able to efficiently screen large compound libraries in a wide variety of matrices. In the case of bioactive mixture libraries, the identification of individual compounds providing the initial bioactivity is rather laborious and time consuming. Commonly used high-throughput screening (HTS) methodologies after mixture fractionation are often only capable of identifying some of the bioactive compounds present.14 Another aspect compromising effective mixture analysis lies in the difficulty of correct matching of dereplicated bioactives in particular fractions with their chemical structure.15,16 To circumvent these problems, literature reports approaches that are based on frontal-affinity chromatography,17 affinity-capturing methods,18 ultrafiltration strategies19 and size-exclusion based methodologies.20 In these cases the analytical principle is based on the affinity capture of bioactive compounds by the target protein followed by a rapid separation step of the bioactive compounds bound to the target protein and the non-binders. After subsequent disruption of the protein-bioactive compound complexes, automated (LC–)MS analysis is used to identify the released bioactive compounds. Disadvantages of these methodologies can be the necessity to chemically modify the protein, to immobilize the protein, the occurrence of non-specific binding and/or the dilution of protein-bioactive-compound complex during separation from non-bound compounds. Also, limitations in separation efficiency can be a severe drawback. Furthermore, accuracy and sensitivity as a result of ion suppression in the mass spectrometer may hamper efficient analysis.
Dynamic protein-affinity chromatography coupled to SPE–LC–MS has been recently developed by us to overcome these problems.13 The methodology uses His-tagged proteins for in-solution affinity binding under native conditions. His-tagged proteins contain polyhistidine-tags (His-tags) often used for affinity purification of recombinant proteins that are expressed in E. coli. Currently, they are the standard in recombinant protein expression and the workhorse in drug discovery screening campaigns. After incubation of the analytes with the His-tagged target protein, the incubated mixture is injected into a fully automated sample pre-preparation system in which the sample passes a Ni2+ -loaded IMAC column allowing efficient trapping of the His-tagged protein with the bioactive compounds bound. A subsequent washing procedure is followed by a disruption step that allows the bioactive compounds to be eluted to a disposable C18-SPE cartridge. After washing the SPE cartridge with LC–MS compatible eluents, the SPE cartridge is switched on-line to LC–MS for identification of the bioactive compounds in the incubated mixture (Figure 3).
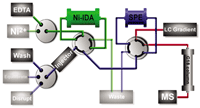
Figure 3: Schematic overview of the dynamic protein affinity chromatographic (DPAC) system. Three separate modules are shown: The DPAC module separating protein-bound ligands from unbound ligands (green). The SPE module enabling a matrix change, washing steps and preconcentration (blue). This module is switched in-line with the gradient LC separation module for ligand elution to MS (red).
The system showed limited non-specific binding in combination with fast and easy regeneration steps in a rather robust analytical valve-switching set-up. Mixture analysis times of 18 min could be achieved resulting in efficient measurement of comprehensive profiles of bioactive compounds in mixtures in a fully automated and label-free way. Typical binding results for a set of test compounds (bioactive compounds and non-binders) including a dose-response curve for affinity profiling and MS spectra for identification are shown in Figure 4. As weak binders (with 100 µM to 1 mM affinity) could be identified and the system, in principle, can be used for other His-tagged proteins in a more or less universal approach, the methodology represents a sensitive and generic tool for bioanalytical affinity profiling of mixtures.
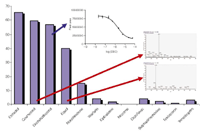
Figure 4: Typical comprehensive data obtained with the DPAC system. Shown here are the maximal percentages of ligands (or non-binders) bound to receptor recalculated from the Bmax when incubated in large excess compared with their Kd values. Lowest affinity binders in the figure are equol and norethindrone. Red arrows point to MS data obtained for the identification and construction of affinity profiles. Blue arrow points to a complete affinity profile for diethylstilbestrol (DES) analysed in a concentrationâresponse manner.
Development of a counter gradient parking system for gradient liquid chromatography with on-line biochemical detection of serine protease inhibitors21
When analysing natural extracts or metabolic mixtures for bioactive compounds, the tedious bioanalytical analysis of the bioactivity for individual compounds in mixtures is usually done after separation, fraction collection and concentration. Apart from being tedious, this process has severe difficulties with correlating the bioactivities in fractions with the corresponding compounds (from MS identification) as the bioactivity density is much lower than the chemical information density. This simply means that in the time frame where an eluted (bioactive) fraction is detected, many compounds are detected in the identity trace. In this area, our group is especially interested in developing new technologies to overcome the problems of normally employed dereplication processes. An efficient strategy involves the on-line coupling of bioassays after LC separation.
This methodology proved to be successful for bioassay formats with fast and sensitive responses thereby providing rapid and accurate bioaffinity data to identity correlations. When using gradient-LC coupled to an on-line bioassay, usually a complex post-column addition of a counter gradient system is needed to maintain a stable and bioassay-compatible eluent composition.22,23 To overcome this problem, we developed a gradient-LC approach in combination with a straightforward counter gradient system.21 The system was able to produce a biocompatible constant solvent composition after the gradient LC elution. It is based on storage of complete gradients in an additional preparative LC column followed by reversed elution and mixing into the effluent from the separation column. With this set-up, no additional LC pumps are needed and enhanced stability is one of the other advantages. The system was applied for the on-line biochemical detection of protease inhibitors. The bioassay is based on the elution of inhibitors into a continuous-flow system where the inhibition of the prototypic conversion of the substrate to a fluorescent product (in a reaction coil) results in negative peaks (Figure 5). It can clearly be seen from the stability of the biochemical response baseline, that during gradient elution, the counter gradient system is adequate in maintaining a constant bioassay compatible concentration of organic modifier.
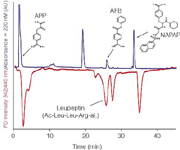
Figure 5: Gradient separation of protease inhibitors with their ability to inhibit trypsin shown in the lower bioactivity chromatogram (red). The upper chromatogram is the UV trace (220 nm) [blue]. Only four inhibitors are depicted. Some high affinity inhibitors are hardly detected in the UV trace, but clearly in the bioactivity trace. Due to the on-line biochemical incubation, there is a time lag between the UV-trace and the bioactivity trace.
Application of hyphenated post-column biochemical assays, high resolution MS and NMR for the characterization and identification of oestrogenic metabolites formed by Cytochromes P450
Biotransformation of drugs often leads to metabolites with enhanced pharmacological or toxicological properties. In current research efforts using an on-line biochemical approach,24 a parallel on-line assay towards the human oestrogen receptor α (hERα) and hERβ subtypes was developed and combined with high resolution MS. Cytochromes P450 (CYPs) were used to metabolize known oestrogenic drugs to generate metabolites with affinity towards hERα and/or hERβ. The CYP incubations were scaled up allowing fractionation and purification using preparative LC and subsequent NMR spectroscopy (1D-1H, 2D-COSY, 2D-NOESY and 1H-13C-HSQC experiments were performed). This resulted in full 3D structure and conformational analysis for the metabolites.
On-line analysis of catalyst performance and identification25
For the discovery and development of novel catalysts, combinatorial synthesis methodologies are used to create libraries of catalysts that have to be screened for catalytic activity. To cope with large pools of potential catalysts, fast screening methods are essential and we developed an analytical methodology to rapidly analyse catalytic performance in an on-line format.25 The first element is the catalyzed multicomponent reaction (MCR) performed in a thermostatted reaction coil. For this, an MCR was used to synthesize 2-imidazoline derivatives. The second element is the MS monitoring of catalyst performance by on-line detection of the products formed by the catalysed reaction (Figure 6). Simultaneous identification of the catalysts by MS can also be performed. Good agreements with traditionally applied bench-scale experiments were obtained. The system can be adapted to other synthetic conversions in a straightforward way by adjustment of the continuous flow (from a conventional LC separation) and the reaction substrates thereby being a potentially strong and general tool for rapid analysis of catalyst performance.
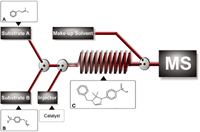
Figure 6: Schematic overview of the on-line analytical system. Two HPLC pumps deliver the imine (substrate A) formed after the condensation of acetone and benzylamine (in the solvent bottle) and the p-nitrobenzylisocyanide (substrate B) to the reaction coil. Homogeneous catalysts are injected into the stream of substrates, then catalysing the reaction to form product C. A third make-up HPLC pump is used to add a water flow that quenches the reaction and increases APCIâMS detector compatibility.
Targeted small molecule biomarkers of toxicity26
Aldehydes are important biomarkers for oxidative stress resulting in lipid peroxidation. The reactive oxygen species responsible for the onset of oxidative stress are produced during metabolic processes and by other factors such as environmental pollutants, UV-light or several diseases. As reactive oxygen species have very short lifetimes in biological matrices, they are usually measured indirectly via biomarkers of radical damage in blood or urine. To enable analysis of aldehydes by LC–MS, derivatization is required. Derivatization with dinitrophenylhydrazine (DNPH) is most widely applied, but suffers from a number of disadvantages. Therefore, we developed a novel, highly sensitive and selective derivatization agent, 4-(2-(trimethylammonio)ethoxy) benzenaminium halide (4-APC), that allows the selective derivatization of aldehydes.26 One of the key features of 4-APC, the incorporation of a permanent positive charge, significantly reduces ionization suppression, increases ionization efficiency and is ideally suited for structure-specific MS–MS analysis of aldehydes in biological samples. Moreover, aldehydes can now be analysed in positive ion mode electrospray MS together with the other two important biomarker classes for oxidative stress, the hydroxytyrosines for protein damage and 8-hydroxy-2'-deoxyguanosine for DNA damage.
The aldehyde derivatization itself is a straightforward one-pot fast reaction under relatively mild conditions. The in-vial derivatized products can be injected directly for LC–MS analysis. Linearity was between 10–500 nM with detection limits of 3–33 nM for the different aldehyde derivatives. Finally, the derivates give characteristic losses of 59 and 87 Da upon fragmentation thereby proving to be an ideal tool for sensitive and specific monitoring of (unknown) aldehydes with MS–MS. Figure 7 shows the typical results obtained for an analysis of aldehydes derivatized with the 4-APC reagent.
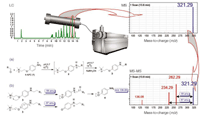
Figure 7: Schematic LC separation of aldehyde derivatives followed by MS and MSâMS analysis of the nonanal derivative (m/z = 321.29). (a) Derivatization scheme: The charged ammonium group allows highly sensitive detection of the derivatives by positive ion mode electrospray MS. (b) Typical confirmatory neutral losses of 59 amu and 87 amu are observed in MSâMS mode. Using high fragmentation energies, a the common oxirane ion (136.08 amu) can also be observed.
Multidimensional Separations27
Conventional separation techniques such as one-dimensional (1D) LC or GC can be a limiting factor in the analysis of complex biological mixtures containing many polar to non-polar compounds with concentrations in the picomolar to millimolar range. Furthermore, diverse physico-chemical properties such as acid–base properties, stability, solubility and detectability are encountered in a sample matrix. A wide variety of LC technologies can be combined into two-dimensional (2D) or multidimensional separation techniques to enhance selectivity in target-compound analysis (e.g., the on-line immunoaffinity chromatography-reversed-phase LC and dynamic protein-affinity chromatography reversed-phase LC techniques discussed above). However, if comprehensive rather than target analysis of complex biological matrices is required, different approaches must be taken. Nowadays, comprehensive detection of (most of the) analytes is becoming more and more important, for example, in systems biology studies. In this respect, comprehensive two-dimensional chromatography (e.g., GC×GC or LC×LC) is a powerful technique with the advantage of simplified automation, improved reproducibility and relatively short analysis times. Whereas, a number of LC×LC separations have been published (e.g., RPLC×RPLC, RPLC×SEC, normal-phase-LC×RPLC, SEC×RPLC) with more or less orthogonality between the two LC steps, none of these combinations appears to be ideal. LC×LC separations have been described as employing different separation principles in the first and second dimension, including, IEC×RPLC and RPLC×IEC. For comprehensive small molecule analysis in combination with MS detection (e.g., metabolic profiling) RPLC×IEC would be less favourable as the IEC generally requires relatively high salt concentrations thus making it less compatible with MS detection. These shortcomings can be overcome by combining RPLC in the first dimension with a mixed-mode column featuring both RPLC and IEC separation mechanisms in the second dimension. Therefore, an automated LC×LC system coupled to MS featuring a 60 min gradient elution on a first-dimension RPLC column with transfer of the eluate in 60 s fractions to a second-dimension mixed-mode cation-IEC/RPLC column was developed.27 The second dimension separation uses isocratic elution based on a step gradient of both organic and ionic modifier (ACN and TFA) resulting in high peak capacities and improved peak resolution of the complete system, without compromising stability and background signals of the detectors used (UV and APCI-MS). The system was developed and validated with a 32-component test mixture comprising compounds with widely differing physico-chemical properties (pKa, polarity measured as logD, ranging from –3.4 to +3.7). The final system is highly flexible and has a broad applicability. Intraday and interday variability of the retention times of the complete system were 0.5% and 0.7%, respectively with good linearity observed for three different compounds with varying polarities. Some preliminary results with (spiked) urine samples finally demonstrated the applicability of the system.
Conclusions
Complete analytical automation implementing on-line digestion procedures prior to or after clean-up steps for protein analysis has proven to be an excellent approach for MS–MS-based identification. Sequence analysis allows the efficient analysis of proteins with large similarities or proteins that are covalently modified because of reactive chemicals or post-translational modifications. Future directions in this area are the development analytical technologies for comprehensive analysis of protein–drug adducts resulting from reactive metabolites.
By combining LC separations with biochemical analysis and MS identification, the selectivity and sensitivity as well as sample throughput are significantly increased for the analysis of complex biochemical mixtures. The on-line integrated techniques allow affinity interaction profiling of protein–protein and protein–ligand interactions. When the integrated biochemical analysis is performed label free, with MS readout, disadvantages such as fluorescent probes with significant affinity for the receptor or the enzyme analysed are circumvented. Furthermore, biochemical analysis can be performed for different biochemical assays in one analysis run as the MS is capable of measuring multiple different probe ligands or substrates simultaneously. The combination of an analytical separation with biochemical assay formats can be divided into three distinct approaches, namely the biochemical interaction before ligand isolation and subsequent (LC–)MS, by using the biochemical interaction during the analytical separation and biochemical interaction analysis after analytical separation strategies. All three procedures have their own distinct advantages and disadvantages in terms of selectivity, specificity, resolution and sensitivity. We are currently thoroughly evaluating and describing these factors for the different strategies for a future review publication. Taken together, with on-line systems combining analytical separations, biochemical analysis and MS identification, high throughputs, reproducibilities and comprehensive bioactivity profiles (e.g., IC50s) with correlating identity information (e.g., polarity, molecular mass) can be achieved for bioactive compounds in mixtures. Future research efforts in this area are directed at the application of these comprehensive analysis tools for bioactive mixtures towards difficult and/or expensive targets. One can think of targets that are very prone to inactivation and/or have slow biochemical assay readouts such as protein kinases, ion channels and GPCRs. For this, it is anticipated that fundamentally different analytical approaches have to be taken to successfully analyse these targets with sufficient sensitivity and specificity.
For complex analytical challenges, multidimensional separations can provide an efficient answer. More specifically, automated LC×LC separations are able to obtain very high peak capacities for biochemical samples as bodily fluids. In current state-of-the art research activities involving systems biology where the "omics" research areas demand analytical methodologies with ever-increasing resolutions, sensitivities and dynamic ranges, these multidimensional LC×LC separations are pivotal. Analytical advances combining different separation mechanisms to obtain high orthogonalities and MS compatibility are not only important in these times, but even form the basis for advances in systems biology by providing new analytical strategies for ever increasingly comprehensive analysis results. Here, we focus our future research activities in development of novel multidimensional LC×LC separations capable of efficiently analysing comprehensive metabolic profiles from diverse body fluids in medical settings.
Hubertus Irth has been professor for bioanalytical chemistry at the VU university since 1999 with a focus on the implementation of biospecific interactions in analytical technologies. In 1997 he founded Kiadis BV (formerly Screentec BV), a spin-off company developing and commercializing novel drug discovery technologies. Currently he is head of the department of Chemistry and Pharmaceutical Sciences. Henk Lingeman is currently the head of the BioMolecular Analysis group. His research programme is mainly focused on the development of automated sample preparation and analysis procedures for the determination of analytes, especially drugs, peptides and proteins — in biochemical and pharmaceutical samples. Wilfried Niessen is professor of bioanalytical mass spectrometry. His research interests involve principles, technology and applications of (hyphenated) mass spectrometry. In 1996, he established a consulting company; hyphen MassSpec that provides international training and consultancy in the area of bioanalytical mass spectrometry. Jeroen Kool started in 2007 as a tenure track assistant professor where his research focus is at the implementation and parallelization of novel bioanalytical techniques designed for comprehensive bioactive mixture analysis towards difficult and/or expensive targets as protein kinases, ion channels and GPCRs.
References
1. J. Carol-Visser et al., J. Chromatogr. B, 870 , 91–97 (2008).
2. J.S. Hoos et al., J. Chromatogr. B, 859 , 147–156 (2007).
3. J.S. Hoos et al., J. Chromatogr. B, 830 , 262–269 (2006).
4. B. Bruyneel et al., Anal. Chem., 79 , 1591–1598 (2007).
5. G. Hopfgartner and E. Varesio, Trends Anal. Chem., 24 , 583–589 (2005).
6. A.R. de Boer et al., Lab. Chip, 5 , 1286–1292 (2005).
7. A.R. de Boer et al., Anal. Chem., 76 , 3155–3161 (2004).
8. T. de Boer et al., J. Pharm. Biomed. Anal., 34 , 671–679 (2004).
9. R.J. Derks et al., Anal. Chem., 75 , 3376–3384 (2003).
10. J. Hirata et al., J. Chromatogr. A, 1081 , 140–144 (2005).
11. A.C. Hogenboom et al., Anal. Chem., 73 , 3816–3823 (2001).
12. J.G. Krabbe et al., J. Chromatogr. A, 1130 , 287–295 (2006).
13. N. Jonker et al., J. Chromatogr. A, 1205 , 71–77 (2008).
14. S. Mouhat et al., Curr. Pharm. Des., 14 , 2503–2518 (2008).
15. F.E. Koehn and G.T. Carter, Nat. Rev. Drug Discov., 4 , 206–220 (2005).
16. K.P. Mishra et al., Biomed. Pharmacother., 62 , 94–98 (2008).
17. W. Ng et al., J. Biomol. Screen, 12 , 167–174 (2007).
18. J.P. Ferrance, J. Chromatogr. A, 1165 , 86–92 (2007).
19. R.B. van Breemen et al., Anal. Chem., 69 , 2159–2164 (1997).
20. I. Muckenschnabel et al., Anal. Biochem., 324 , 241–249 (2004).
21. N.H. Schebb et al., Anal. Chem., 80 , 6764–6772 (2008).
22. J. Kool et al., J. Biomol. Screen, 12 , 396–405 (2007).
23. J. Kool et al., Drug Metab. Dispos., 35 , 640–648 (2007).
24. J. Kool et al., J. Med. Chem., 49 , 3287–3292 (2006).
25. C.T. Martha et al., Anal. Chem., 80 , 7121–7127 (2008).
26. M. Eggink et al., Anal. Chem., 80 , 9042–9051 (2008).
27. M. Eggink et al., J. Chromatogr. A, 1188 , 216–226 (2008).

Thermodynamic Insights into Organic Solvent Extraction for Chemical Analysis of Medical Devices
April 16th 2025A new study, published by a researcher from Chemical Characterization Solutions in Minnesota, explored a new approach for sample preparation for the chemical characterization of medical devices.
Sorbonne Researchers Develop Miniaturized GC Detector for VOC Analysis
April 16th 2025A team of scientists from the Paris university developed and optimized MAVERIC, a miniaturized and autonomous gas chromatography (GC) system coupled to a nano-gravimetric detector (NGD) based on a NEMS (nano-electromechanical-system) resonator.
Common Challenges in Nitrosamine Analysis: An LCGC International Peer Exchange
April 15th 2025A recent roundtable discussion featuring Aloka Srinivasan of Raaha, Mayank Bhanti of the United States Pharmacopeia (USP), and Amber Burch of Purisys discussed the challenges surrounding nitrosamine analysis in pharmaceuticals.
Extracting Estrogenic Hormones Using Rotating Disk and Modified Clays
April 14th 2025University of Caldas and University of Chile researchers extracted estrogenic hormones from wastewater samples using rotating disk sorption extraction. After extraction, the concentrated analytes were measured using liquid chromatography coupled with photodiode array detection (HPLC-PDA).
Silvia Radenkovic on Building Connections in the Scientific Community
April 11th 2025In the second part of our conversation with Silvia Radenkovic, she shares insights into her involvement in scientific organizations and offers advice for young scientists looking to engage more in scientific organizations.
















