UHPLC Seperation and Detection of Bisphenol A (BPA) in Plastics
PerkinElmer Application Note
Roberto Troiano and William Goodman, PerkinElmer Inc., Waltham, Massachusetts, USA.
Introduction
BPA or bisphenol A has become well known over the past year as concerns for its effect on human health and well being have been raised. The concerns over BPA in plastics began with baby bottles and spread to include other types of bottles and toys.
BPA is used in the production of two very common polymers: PVC and polycarbonate. As a result of the health concerns over human exposure to BPA, this molecule is now monitored in specific products, including baby bottles and other children's products. Simple and robust test methods are needed to determine the presence and amount of BPA in plastic materials. This paper will present the extraction and HPLC analysis of plastic children's products for BPA.
Experimental
The study presented here includes extraction of BPA from a toy matrix and analysis with UHPLC. The extraction procedure is intended to simulate the contact routes through which children are likely to encounter BPA. Two different extraction techniques were used to analyse BPA in samples (30 g sample used for each extraction). The first extraction method immersed the sample in 1 L of water, at 40 °C for 24 hours (EN 14372). The second immersed the sample with 1 L HCl (0.07 M) at 37 °C for 2 hours. Following extraction the samples were analysed with a PerkinElmer Flexar FX-10 UHPLC system including a PerkinElmer Series 200a fluorescence detector. The separation was performed on a Brownlee validated C8 column.
Results
The BPA analysed with the given LC conditions eluted at 5.43 mins. The UHPLC system was calibrated across a range of 1–50 ppb (μg/L) BPA. The limit of quantification (LOQ) for BPA with the method presented here is 1 ppb. The signal-to-noise at the LOQ is approximately 10:1. The response across the calibration range fit a linear calibration with an r2 value of 0.9993. Blanks analysed between standards and samples showed the system was free from any BPA contamination or carryover.
BPA in the extracts of the toy samples were quantified using the calibration curve generated during standard analysis. Figure 1 shows the chromatogram of the water extract of the toy dwarf sample.
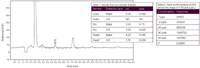
Figure 1: Analysis of toy dwarf for BPA using water.
The extraction procedure, which heated the toy for 24 hours in water at 40 °C, extracted a significantly higher amount of BPA from the matrix than the extraction in acid. BPA was found in all three water extractions within the calibration range of the standard curve.
Conclusion
As health concerns over exposure to BPA are raised, its analysis in plastics is becoming very important. The PerkinElmer Flexar FX-10 UHPLC system provides a sensitive and robust platform for this analysis. Demonstrated here was a calibration of BPA across a range of 1–50 ppb with a chromatographic run time of less than 10 minutes. This analysis was applied to three toy samples and BPA was identified in each sample.

PerkinElmer Inc.
940 Winter Street, Waltham, MAssachusetts 02451, USA
tel. +1 800 762 4000
Website: www.perkinelmer.com

New Method Explored for the Detection of CECs in Crops Irrigated with Contaminated Water
April 30th 2025This new study presents a validated QuEChERS–LC-MS/MS method for detecting eight persistent, mobile, and toxic substances in escarole, tomatoes, and tomato leaves irrigated with contaminated water.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)












