High Resolution UPLC Analysis of 2-AB Labelled Glycans
Waters Application Note
Beth Gillece-Castro, Kim van Tran, Jonathan E. Turner, Thomas E. Wheat and Diane M. Diehl, Waters Corp., Milford, Massachusetts, USA.
Introduction
Glycosylation is a post-translational modification process that can significantly affect the biological properties of therapeutic proteins. The successful analysis of N- and O-linked glycan structures requires the ability to reproducibly resolve complex mixtures of isomeric oligosaccharides. Hydrophilic-interaction liquid chromatography (HILIC) is a powerful technique for separating a wide range of glycan structures. If the glycans have been labelled with a tag such as 2-amino benzamide (2-AB), the fluorescence can be monitored and used for relative quantification. The technique of Ultra Performance Liquid Chromatography (UPLC) is appropriate for such a difficult analytical problem. UPLC is based on the use of columns packed with very small particles and instruments optimized for use with these high resolution columns. The ACQUITY UPLC BEH Glycan column was specifically designed for use with Waters ACQUITY UPLC system. This analytical solution gives improved resolution, sensitivity and speed compared to HPLC techniques.
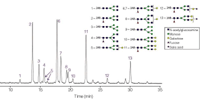
Figure 1: The N-linked glycans from pooled human IgG include high mannose, neutral and sialylated complex structures. The chromatogram shows 35 minutes of a one hour run.
UPLC conditions
Figure 1 sample: 15 pmol 2-AB-(human IgG library)*
Figure 2 sample: 15 pmol 2-AB-(bovine fetuin library)*
Column: ACQUITY UPLC BEH glycan 1.7μm, 2.1 × 150 mm
Eluent A: 100 mM ammonium formate, pH 4.5
Eluent B: Acetonitrile
Figure 1 gradient: 75–50% B over 46.5 min
Figure 2 gradient: 75–60% B over 46.5 min
Temperature: 60 °C
*ProZyme, San Leandro, California, USA
Results
The first chromatogram shows the separation of N-linked oligosaccharides released from human IgG and labelled with 2-AB. This sample naturally includes a mixture of high mannose, complex, hybrid and sialylated glycans. Because the mixture contains a wide range of glycan classes, this sample was chosen for use as the QC standard for developing and manufacturing this column. This test ensures reproducible selectivity and resolution.
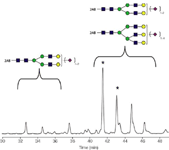
Figure 2: Analysis of 2-AB (Bovine Fetuin N-linked glycan library). Highlighted(*) are multiple peaks with a single molecular weight corresponding to disialylated biantennary glycans.
The chromatographic separation of all of the major oligosaccharides in the mixture, G0F, G1F, G2F and sialylated G2F is shown. The resolution of the structure G0F (peak 2) from the structure Man5 (peak 3) is particularly important. The two isomers of G1F are also completely resolved and the significance of their relative abundance may now be investigated.
The second chromatogram shows the separation of glycans released from bovine fetuin. These oligosaccharides include differing numbers of branch antennae and differing numbers of charged, acidic sialic acid termini. A challenging pair of isomers that are resolved (*) are triantennary glycans with two sialic acid residues and they elute at 41.4 and 43.1 minutes.
Conclusion
Glycan separation technology columns, when operated with the ACQUITY UPLC instrument and FLR detector provide a highly-resolving, reproducible, rapid method for profiling glycans. The improved analysis provides more certain identification and reliable quantification.
For complete details, visit www.waters.com/31123
© 2009 Waters Corporation. Waters, The Science of What's Possible, UPLC, and ACQUITY UPLC are trademarks of Waters Corporation.

Waters Corporation
34 Maple Street, Milford, Massachusetts 01757, USA
tel. +1 508 478 2000 fax +1 508 482 3605
Website: www.waters.com

Detecting Hyper-Fast Chromatographic Peaks Using Ion Mobility Spectrometry
May 6th 2025Ion mobility spectrometers can detect trace compounds quickly, though they can face various issues with detecting certain peaks. University of Hannover scientists created a new system for resolving hyper-fast gas chromatography (GC) peaks.
University of Oklahoma and UC Davis Researchers Probe Lipidomic Profiles with RP-LC–HRMS/MS
May 6th 2025A joint study between the University of Oklahoma Health Sciences Center (Oklahoma City, Oklahoma) and the UC Davis West Coast Metabolomics Center (Davis, California) identified differentially regulated lipids in type 2 diabetes (T2D) and obesity through the application of reversed-phase liquid chromatography-accurate mass tandem mass spectrometry (RP-LC-accurate MS/MS).
Automated Sample Preparation (ISO 20122) for MOSH/MOAH in Seasoning Oils
May 6th 2025This work presents an Automated Sample Preparation procedure for MOSH/MOAH analysis of Seasoning Oils. We compare results from a manual epoxidation procedure compliant with DIN 16995 with results based on fully automated sample preparation (epoxidation and saponification) compliant with ISO 20122. In both cases, online clean-up via activated aluminum oxide (AlOx) are used to remove interfering n-alkanes from the MOSH fraction during the HPLC run. Automated data evaluation using a dedicated software (GERSTEL ChroMOH) is presented.

.png&w=3840&q=75)

.png&w=3840&q=75)



.png&w=3840&q=75)



.png&w=3840&q=75)












