Highlights of HPLC 2014
LCGC Asia Pacific
This year's HPLC symposium was held in New Orleans, Louisiana, USA. In this review, Ron Majors covers HPLC and UHPLC column and sample preparation highlights, summarizes the awards presented, and reviews the overall liquid-phase chromatography trends.
HPLC 2014 was held 11–15 May in New Orleans, Louisiana, USA, for the first time and much earlier than normal. This instalment of "Column Watch" covers some of the technology and application advances presented at HPLC 2014. We review the overall liquid-phase chromatographic trends, summarize the awards presented, and discuss the column technology highlights observed at the symposium.
The 41st International Symposium on High Performance Liquid Phase Separations and Related Techniques, which alternates between Europe and North America, with occasional side meetings in Australia and Asia, was held 11–15 May in New Orleans, Louisiana — its first time in the southern part of the United States. More affectionately known as HPLC 2014, the symposium is the premier scientific event for bringing together the myriad techniques related to separations in liquid and supercritical fluid media. Chaired by Professor J. Michael Ramsey of the University of North Carolina at Chapel Hill, USA, with the able assistance of the symposium organizer Professor Edward Yeung of Iowa State University, USA, and Janet Cunningham of Barr Enterprises, HPLC 2014 assembled 770 delegates from 39 different countries around the world. This number included vendor representatives from 44 exhibitors for the three-day plus instrument, software, and consumables exhibition. The number of conferees was just over half of the attendance at HPLC 2013 in Amsterdam, The Netherlands, and in line with the HPLC 2012 symposium held in Anaheim, California, USA. For some reason, the number of delegates for the United States version of this meeting has fallen off in recent times, perhaps analogous to the falloff in other analytical symposia such as Pittcon. However, other meetings such as the American Society for Mass Spectrometry (ASMS) Conference on Mass Spectrometry and Allied Topics seem to be gathering steam, undoubtedly driven by the recent surge in the use of mass spectrometry (MS) detection in chromatography and other analytical techniques.
The venue for HPLC 2014 was the New Orleans Hilton located right on the Mississippi River close to the convention centre where Pittcon has been held many times. The five-day plus event had a total of 155 oral presentation in plenary talks and mostly in three parallel sessions, which made it a bit difficult to cover topics of interest that often ran at the same time. Fortunately, all three lecture halls were within a few feet of each other so getting from one session to another didn't pose much of a problem. At HPLC a total of 425 posters were presented in sessions with 25 different themes. Posters were up for the entire symposium so they could be viewed at almost any time of the day. With an ample social event schedule including three receptions and a symposium dinner and party, 10 vendor workshops, eight tutorial educational sessions, and five short courses (held during the previous weekend), attendees had their hands full deciding how to allocate their time. The tutorials were particularly well attended, some with standing room only, and covered current topics such as troubleshooting method development, polymeric monoliths, microfluidics, column myths, ultrahigh-pressure liquid chromatography (UHPLC) theory and practice, the effect of dwell volume, ion chromatography versus electrophoresis, and new Food and Drug Administration (FDA) regulations affecting high performance liquid chromatography (HPLC).
In this instalment, I present some scientific highlights of HPLC 2014. This report also covers the various awards and honourary sessions that took place. Since it was virtually impossible for one person to adequately cover all oral and poster papers, my coverage will reflect a somewhat personal bias.
Trends in Liquid-Phase Technology and Techniques
Obviously, HPLC was the predominant technology in the technical sessions at the symposium, but sample preparation, the use of electrophoretic techniques (mostly in a capillary format), and an increase in supercritical fluid chromatography (SFC) papers were strongly evident. From a perusal of the poster and oral presentation abstracts, I broke down some of the major areas of coverage in this year's symposium and listed them in tables. These tables are useful to spot trends in the technology and new application areas for liquid-phase separations that were introduced in this series.
Table 1 provides a rough breakdown of the coverage of liquid-phase technology and techniques in the separation sciences. Compared to HPLC 2013, some slight shifts in technology emphasis were noted. Again this year, new developments in column technology led the pack with oral presentations and poster papers dealing with many new phases and formats. However, compared to previous symposia in the series, the percentage of column-related talks actually dropped from a third of all presentations to a quarter of all presentations. Surprisingly, nearly 40% of the columns' papers dealt with monoliths, with polymeric monolith coverage nearly 2:1 over silica-based monolith talks and posters. In the future, silica monolith coverage may grow because the patents are winding down and perhaps new companies may investigate the technology. Although not yet considered a commercial success, research interest, especially in academia, in monolith technology is still running high. The polymeric monolith segment is less covered by intellectual property rights than the silica monolith segment. Silica gel-based monoliths are seeing their second generation, and maybe a third generation, of commercial products with better efficiency, but slightly higher pressure drops because of the change in the macropore–mesopore domain ratios. Still, the silica monoliths are only available from one source. However, a continuation of new developments in polymeric monoliths devoted to the separation of small molecules has shown improvements in column efficiency. Originally, silica-based monoliths were considered to be best for small molecules and polymeric monoliths were thought to be optimum for large biomolecules only. Those beliefs are beginning to change as silica monoliths are being developed for large molecules and polymeric monoliths for small-molecule separations.
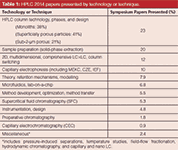
Table 1: HPLC 2014 papers presented by technology or technique.
Three other "hot" areas in column technology this year were:
- Continuing with the observation made in my recent Pittcon article (1), superficially porous packings (SPPs, also referred to as shell particles, poroshell, core–shell, and fused-core packings) that rival the sub-2-µm particles in terms of column efficiency, but with substantially lower pressure drops are now the hottest area in HPLC and UHPLC. The poster and oral papers referring to SPPs outnumbered those devoted to sub-2-µm totally porous particles. The availability of sub-2-µm SPPs and up to 5-µm SPPs have expanded the use of these special particles to mainstream HPLC as well as UHPLC applications. At HPLC 2014, there were reports of even smaller SPPs than those currently commercially available.
- Papers on two-dimensional (2D) and multidimensional chromatography doubled compared to last year's meeting. The technique is becoming more mainstream since major LC instrument companies now have easy-to-use accessories that provide accurate and rapid column switching and LC×LC (comprehensive LC) capabilities. For LC×LC, acceptance has also been brought about by the availability of more orthogonal stationary phases and column configurations such as short, fast SPP and monolithic columns for the second dimension. The 2D techniques are mainly useful when complex samples are encountered; food analysis was the most popular area for applications this year.
- Microfluidics, microchips, and nanochannels were all part of a continuing theme both in oral and poster sessions. Undoubtedly, the strong interests of the symposium chair in this discipline probably accounted for a slight bias in acceptance of papers looking at smaller dimension columns and instrument design to accommodate them. One could also lump in micro- and nano-LC columns into this category that I didn't segment separately. All of these approaches not only result in solvent and sample savings, lower dispersion, and higher sensitivity, but also easier interfacing into detectors such as MS detectors. One paper even showed the use of the flame ionization detector, which can cope with a low solvent flow rate.
In the columns area, I broke down the modes being used by HPLC 2014 attendees (see Table 2). As always, on a relative basis, reversed-phase LC again dominated the usage (43% of all papers) with hydrophilic-interaction chromatography (HILIC) maintaining its position at a distant number two. HILIC serves as a separation technique for polar analytes that are weakly retained by reversed-phase chromatography. The number of chiral separation papers showed a strong number three with SFC applications a driving force.
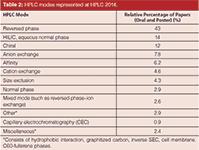
Table 2: HPLC modes represented at HPLC 2014.
Sample preparation technologies were well represented in the poster papers, but only five oral presentations were presented that had a sample preparation theme. No organized sessions were devoted to sample preparation this year. For poster presentations, the most prominent sample preparation subjects were solid-phase extraction (SPE), on-column digestion of proteins using immobilized enzymes, protein precipitation for analyzing drugs and metabolites in biological fluids, and filtration. On-line SPE, related to the column switching approach discussed above, was the subject of several posters. New instrumentation accessories have made on-line SPE easier to perform.
Electrodriven separation techniques (such as capillary electrophoresis [CE], capillary zone electrophoresis [CZE], micellar electrokinetic chromatography [MEKC], and isoelectric focusing [IEF]) grew this year with a strong showing in both oral and poster papers with many applications papers depicting great strides in interfacing to MS. A continued lack of interest in capillary electrochromatography (CEC) was noted with only four presentations at HPLC 2014.
Areas of Application
Table 3 is a brief breakdown of the most popular application areas reported at HPLC 2014. This year, I finely divided the applications areas, but again oral and poster presentations on life science topics dominated all applications. The various "omics" (for example, metabolomics, proteomics, and lipidomics) were out in front with post-translational modifications (such as glycosylated and phosphorylated proteins), monoclonal antibodies, and the search for biomarkers as the major fields of study. The techniques LC–MS and LC–MS–MS are an absolute requirement for these studies; hence, there were an overwhelming number of papers with these MS technologies presented at this meeting.
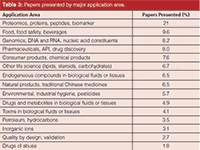
Table 3: Papers presented by major application area.
Pharmaceutical and biopharmaceutical companies are still the most prolific users of HPLC and UHPLC. If one adds up all the areas in Table 2 related to pharmaceuticals, it would be the second biggest application category, followed by food and food safety applications. Most of the other application areas were roughly the same as in previous years (2,3).
Awards and Honours at HPLC 2014
The HPLC meetings have become the venue for chromatography awards presented by various groups for best posters, best oral presentation by a young investigator, and awards from the Chromatographic Society. This year a new award for significant contributions by industrial chromatographers was added to the mix.
Poster Sessions and Best Poster Awards: The mainstay of HPLC 2014 was the poster sessions where more detailed applications and methodology studies were reported, often in very specific areas, and face-to-face discussions with the authors were conducted. Fortunately, many of the poster authors were kind enough to provide small reproductions of their poster papers that could be taken for later perusal. Some authors collected business cards and addresses for sending poster reprints by mail or e-mail. Compared to HPLC 2013 (1), the number of posters was greatly decreased. Only about half of the poster presenters elected to have their poster evaluated for the Best Poster Contest, which made the job a bit easier for the 31 reviewers that were assigned to the Poster Committee. The posters were up for four days, which gave viewers plenty of time to find them. It appeared that there were fewer "no shows" than had been observed in previous HPLC symposia.
The Poster Committee Chairpersons were Monika Dittman of Agilent Technologies and Chris Pohl of Thermo Fisher Scientific. The Poster Committee devoted a great deal of time and thought to the job at hand. They worked very hard to narrow down the collection of posters by the end of the third day to 17 finalists whose posters were identified with a Finalist placard. From these 17 finalists, reviewers chose the top 10 winners by the Thursday afternoon of the symposium. The selection criteria were based on three factors: Inspiration (creativity, newness, uniqueness, originality); transpiration (experimental execution; completeness of work); and presentation (overall readability, visual impression, author's explanation). Winning posters were viewed by all of the final committee jurors.
The Best Poster Awards, sponsored by Agilent Technologies, were announced at the closing session on Thursday afternoon. For HPLC 2014, the top three Best Poster Award winners (along with their affiliation and title of their poster) are shown in Figure 1. There was a tie for third place between two posters, which was a first since poster prizes started being awarded. Each winner received a cash prize. Because of space limitations, detailed technical coverage of each of the award-winning posters cannot be provided. It suffices to say that all the winners and finalists should be proud of their accomplishments since they represent the top 1% of all posters presented at HPLC 2014.

Figure 1: Top three best poster awards, sponsored by Agilent Technologies. From left to right: Dr. Monika Dittman of Agilent Technologies, award presenter; third place winner (tied): Alexander Siegle, Ruprecht-Karls-Universität Heidelberg in Heidelberg, Germany, "Hadamard Transform Multiplexing LC: Boosting Throughput and Sensitivity of HPLC"; third place winner (tied): Suresh Kumar, Brigham Young University in Provo, Utah, USA, "An Integrated Microfluidic-based System for Complete Analysis of Preterm Birth Biomarkers"; second place winner: Dina Lakayan, VU University Amsterdam in Amsterdam, The Netherlands, "Hyphenation of Surface Plasmon Resonance optical sensing to size-exclusion chromatography for analysis of antibody preparations"; first place winner, Stephenen Groskreutz, University of Pittsburgh, Pittsburgh, Pennsylvania, USA, "Modelling Temperature Assisted Solute Focusing in Capillary High Performance Liquid Chromatography." (Photo courtesy of Martin Gilar, Waters.)
Horváth Award: For the ninth year in a row, the Horváth Award sessions, named for the late Professor Csaba Horváth, one of the founders of this series and a mentor of young scientists, were featured. This award, supported by HPLC Inc. a nonprofit group under the guidance of the Permanent Scientific Committee was established for young scientists in the separation sciences under the age of 35. The award, based on the best oral lecture presented in the Horváth sessions, was selected by a jury named by the Permanent Scientific Committee and consists of a cash prize, an invitation for an oral presentation at HPLC 2015, and a crystal trophy. This year there were seven nominees all with strong research credentials.
The winner of the 2014 Horváth Award was William Black (Figure 2) of the University of North Carolina at Chapel Hill, North Carolina, USA, marking the second year in a row that the award winner was from this institution. The title of his oral presentation was "Integrating Solid-Phase Extraction with Microchip Capillary Electrophoresis — Electrospray Ionization". With limited preconcentration possibilities for CE and with disruptions in electroosmotic flow and band broadening introduced by conventional on-line SPE techniques, Black described a microfluidic platform that was a promising candidate for an integrated CE system. Microfluidic technology is well suited towards integrating multiple functionalities and can precisely manipulate small volumes with zero dead volume. In addition, lab-on-a-chip technologies are amenable to automation, resulting in improved reproducibility and throughput. In his study, a novel design is presented that couples microfluidic SPE with microchip CE–electrospray ionization (ESI) with the goal of a fully integrated analysis system combining the sample processing power of SPE with the speed and separation performance of CE. The design was reproducible (migration times %RSD = 0.5), required low sample consumption (25 fmole loaded on chip), and showed a 150-fold improvement in sensitivity while maintaining high separation efficiency and MS compatibility. Furthermore, this design is potentially adaptable to many different applications by utilizing alternate stationary phases, resulting in a fast, automatable, fully integrated universal platform for sample analysis. Future work will be the addition of alternate sample prep techniques such as on-line desalting, integration with hydrogen–deuterium exchange MS, and improvement in analyte recovery, especially for low k' compounds.

Figure 2: William Black of the University of North Carolina, Chapel Hill, North Carolina, USA, winner of the 2014 Csaba Horvath Young Scientist Award. (Photo courtesy of Martin Gilar, Waters.)
Chromatographic Society Awards: In 1978, Nobel Prize winner Professor A.J.P. Martin gave permission for his name to be associated with the "Martin Medal." The Martin Medal is awarded to scientists who have made outstanding contributions to the advancement of separation science.
At HPLC 2014, the Martin Medal was awarded to Professor Nobuo Tanaka, retired from Kyoto Institute of Technology in 2009, GL Sciences (2009–2013) in Tokyo and, most recently, a visiting professor with the University of California, Davis, California, USA. Professor Tanaka's research is truly multidisciplinary and internationally acclaimed. His work spans research on highly selective stationary phases, isotope separation and separations based on isotopic chirality, separation mechanism elucidation, multidimensional separations, and biological separations. Other notable areas of research include his seminal work on pressure-induced retention changes in reversed-phase LC and stationary phase characterization; and he was also a major contributor in the area of monolithic silica capillary columns for LC and CEC. He contributed to the development of the monolithic silica rod column that was commercialized by Merck in Germany. Tanaka has received numerous awards reflecting his outstanding contributions to the chromatographic sciences.
Although the award ceremony will take place at another chromatography meeting, the Jubilee Medal winner for 2014 is Prof. Michael Lammerhofer of the University of Tubingen, Germany. This award is presented to an up-and-coming young separation scientist. Some of Dr. Lammerhofer's cited contributions were those in chiral stationary phases, metabolomics, and plasmid DNA analysis.
Uwe Neue Award: Uwe Neue, who passed away more than three years ago, was an industrial chemist working for Waters who gained international respect for his contributions to the field of separation science, especially for work that turned into commercial products. The Uwe Neue Award, sponsored by Waters, is directed to scientists, like Uwe, who have had great careers in industry while contributing to the further development of chromatography. The inaugural winner announced at the last HPLC 2013 meeting was Jack Kirkland, and this year's winner was Gerard Rozing, now retired from Agilent Technologies (see Figure 3). Rozing is widely known and recognized as an outstanding researcher for his work that bridges fundamental science with technical solutions in a commercial environment. He worked in industry for more than 30 years before retiring in 2012 to become a consultant for Agilent Technologies and other organizations. He has authored more than 57 publications and holds six US patents.

Figure 3: The Uwe Neue Award presented by Waters. From left to right: Martin Gilar of Waters; Jack Kirkland of Advanced Materials Technology (2013 winner); and Gerard Rozing, retired, Agilent Technologies (2014 winner). (Photo courtesy of Martin Gilar, Waters.)
On his personal webpage, Rozing stated "It is always a great honour to become recognized through an award. But I feel deeply honoured by this award since it is named after Dr. Uwe Neue, a contemporary peer with whom I shared a common career pathway in many aspects. I met Uwe the first time some 25 years ago during one of the HPLC symposium series and many times since then. I have been impressed with his ability to maintain his scientific integrity in a for-profit-organization environment where unlike in academia, the science has to compromise with engineering, marketing, finance, sales, and manufacturing functions." In his address, Gerard thanked the Permanent Scientific Committee for his selection, Waters for their sponsorship, and his colleagues at Agilent for their help and support over the years. In particular, he thanked Peter Hupe, the father of Agilent's liquid-phase separations business and cofounder of this HPLC symposium series, for his part in being Rozing's role model. In addition, he also thanked his friend and career-long manager, Fred Strohmeier, currently senior vice-president of Agilent, who gave him this role and freedom to pursue his more recent career in managing university relations and external collaborations.
Rozing's Award lecture was entitled "Current and Future Perspectives on UHPLC; Requirements for Improved Abilities and Functionality". In his lecture, he first discussed the big changes that occurred a decade ago with the evolution of HPLC to UHPLC driven by the increased pressure requirements of sub-2-µm packings and the advent of high pressure solvent delivery systems that preserved the efficiency gains with the smaller particles. He then speculated as to whether increased pressure beyond current usage will deliver even more chromatographic performance. He concluded that kinetic optimization predicts that the maximum plate number achievable does not increase proportionally with pressure, but that increased pressure will result in a shorter time to obtain the required plate number. However, such inferences from kinetic plot data apply only if H is independent of column length; physical and chemical properties of solvent and solute, particle properties, and column dimensions are independent of pressure; and frictional heating will be negligible. However, we now know that high pressure does affect all of these parameters, but not independently. Rozing then went on to illustrate how pressure affects each of these parameters and presents some challenges in future system design. He presented some alternative ideas such as constant pressure operation and the increased use of multidimensional LC to address the constraints placed on systems and columns by higher pressure operation. For a copy of his complete presentation, go to his website: www.rozing.com.
New Column Technology Highlights
Many oral, poster, and tutorial sessions were devoted to stationary phases and column technology, always topics of high interest at this series of meetings.
Monoliths: As mentioned earlier, in the columns' area, monoliths received a great deal of attention at HPLC 2014. Two oral sessions and one poster session were devoted to monoliths, although monolith papers were scattered throughout the oral and poster sessions. Monolith columns have long been desirable since they exhibit high permeability and low pressure drop (because of increased bed porosity), show reasonable separation efficiencies, have the absence of frits to confine the packing material, are easy to fabricate, and nowadays can be made fairly reproducibly. Although this technology has been around for several years, as a routine tool it has yet to see widespread acceptance on the commercial side, but improvements continue to be made for both polymer- and silica-based monoliths.
In his opening keynote lecture on Monday morning, Professor Frantisek Svec of the Molecular Foundry at Lawrence Berkeley National Laboratory summarized the work of the last two decades of polymeric monolith development, much of which was performed in his own laboratories. His first publication in this area appeared in 1992 (4) and was based on poly(glycidyl methacrylate-co-ethylene dimethacrylate) monoliths. The initial work involved single-step polymerization where the active functionalities were found throughout the monolith but some of these groups were not accessible. Each new monolith chemistry required individualized optimization. The next stage in monolith development involved chemical modification where accessible functionalities could be found throughout the monolith. Further improvement in monolith synthesis was the use of photografting to apply a monolayer of functionalities only on the pore surface. This approach required one-time optimization. A two-step (or multistep) process was used for UV-transparent monomers. An example of the chemistry applied here were the photografted poly(butylmethacrylate-co-ethylene dimethacrylate) monoliths. The group next explored the attachment of nanoparticles with the desired functionality to the pore surface. The surface of the monolith required prefunctionalization before adding the nanoparticles. Their initial study involved the modification of the monolith with functional latex nanoparticles. After these nanoparticles were attached, they formed a very stable, reliable ion-exchange phase.
Recently, Svec and his group began to work with metallic nanoparticles. In the first studies, gold nanoparticles (15 nm diameter) were used. These were found to be quite selective for thiol groups which allowed for a stable, universal ligand that could be used to pull out thiol-containing proteins and peptides from solution. The thiol compounds can be exchanged, thus making the gold nanoparticle on monolith a universal intermediate ligand that enables the surface chemistry to be changed via the attachment of different thiol-containing compounds with desired functionalities. He showed a separation of proteins by reversed-phase chromatography on a HSC18 functionality. The HSC18 was then replaced with an –SO3 containing thiol and used in an ion-exchange mode thereby reversing the elution order. He went on to further exploit this technology by using the gold nanoparticles with two different thiols to provide a mixed-mode stationary phase, one mode with a reversed-phase moiety and the other with a carboxyl functionality. To keep the costs down, Svec is now replacing the gold nanoparticles with silver nanoparticles. The silver also interacts with the thiol groups. The silver–monolith conjugate can now be used as a scavenger for compounds such as iodine and iodide contained in iodinated organic compounds, especially useful for radioactive iodine removal from reaction mixtures, thereby generating a closed solid radioactive waste for easier disposal.
Later in the day, Emily Hilder of the University of Tasmania continued the discussion on the preparation and characterization of porous polymer monoliths for chromatography. She and her colleagues have been focusing on trying to improve the reproducibility of polymeric monoliths by investigating the degree of bed homogeneity using novel polymerization methods. She reviewed a number of the approaches to form ordered porous polymers, such as cryopolymerization using unidirectional freezing, the incorporation of nanoparticles during the polymerization process that improved structural homogeneity, polymerized high internal phase emulsions, and medium internal phase emulsions. They also investigated a range of different emulsion stabilizers including amphiphilic block co-polymers, surfactants, as well as various nanoparticles such as charged ones. The results of the various approaches were studied by scanning transmission x-ray microscopy, which provides high-resolution information about the spatial distribution of chemical components within these monolithic materials. The unidirectional freezing method gave very ordered structures which could be changed by varying the monomer concentration and immersion speed that affected pore size.
A number of other papers dealt with the synthesis of polymeric monoliths for specific tasks. Polymeric monoliths have long been thought to be best suited for macromolecules, but there has been ongoing work on the production of polymer monoliths for small molecules. One research group at the University of Pardubice in the Czech Republic has investigated short thermal polymerization times for the preparation of lauryl methacrylate-co-tetraethyleneglycol dimethacrylate monolithic scaffolds that underwent a secondary polymerization with a zwitterionic monomer and crosslinker to form a HILIC phase. The polymeric monolith was synthesized inside of a 0.32-mm i.d. fused-silica capillary and the column showed 55,000 plates/m for thiourea. Another poster paper studied a similar reactive polyacrylate monolith that was derivatized inside a fused silica capillary resulting in a column also used for HILIC as well as a support for CEC. A group from Saudi Arabia used an in situ polymerization procedure using itaconic anhydride as a monomer and ethylene dimethacrylate as a crosslinker. The itaconic anhydride enabled post-polymerization modification of the monolith column via amidation with a suitable amine. Using octadecylamine and benzylamine as post-polymerization reactants allowed the generation of reversed phase functionality, one with an alkyl (C18) and the other with an aromatic benzene ring.
Silica monoliths were also covered in both oral and poster presentations. Of course, these have been around as commercial products for a long time but researchers continue to work to improve them.
Merck Millipore's Karen Cabrera presented an oral talk about modifications that they have made on their silica monoliths to make them more suitable for large biomolecules. By developing a third-generation monolith with a bimodal pore structure (for example, 2-µm macropores and 30-nm mesopores), the column was found to provide good performance for proteins and antibodies. Separations could be performed within 3–4 min with no carryover. Furthermore, the monolith could be derivatized with 3-glycidyloxypropylsilane, thereby obtaining an epoxide surface enabling chemical immobilization of proteins. Enzymes such as trypsin and affinity phases such as protein A could be immobilized. The trypsin column was coupled in front of an LC column and used for on-column protein enzymatic digestion followed by separation. The protein A phase could be used to quantitatively measure IgG from mixtures. In a poster paper, the same group showed how dirty samples from the food and pharmaceutical industries can be directly injected onto a monolithic silica column and it suffers no ill effects. The reason is that the column doesn't require frits and the unique structure of the stationary phase with flow-through pores provides a high permeability compared to a typical packed LC column. Of course, samples have to be filtered to remove particulates, but spiked samples such as homogenized porcine kidney, a soy drink, and human urine were successfully analyzed by LC–MS. In an interesting poster from Kyoto University in Kyoto, Japan, the author Takuya Kubo immobilized carbon-based nanomaterials (such as graphene, fullerene, carbon nanotubes) to the walls of a fused silica column and to a silica monolithic capillary using a photothermal reactive agent, perfluorophenyl azide. The phases were used for the baseline separation of polycyclic aromatic hydrocarbons with the modified monolith providing a better separation than the wall-coated version. A titanium oxide capillary column–based monolith was developed using the sol-gel process by the Carol Collins group at the University of Campinas in Campinas, Brazil.
The 2014 Martin Gold Medalist Nobuo Tanaka, one of the world experts in silica monoliths, presented a paper in one of the monolith sessions where he spent most of his time discussing extracolumn effects with both small monolithic silica and packed silica capillary columns, the latter with a superficially porous support and a 2-µm C18 totally porous packing material. Basically, it was concluded that for a 50 mm × 1 mm column, unless one has a nanoLC or a very well optimized UHPLC system, it is very difficult to achieve the full efficiency of any column. Columns with smaller internal diameters do not pack as efficiently as columns with larger internal diameters, and some of the efficiency loss is because of the looser packing density. The effect is worst for early eluted peaks. Monolithic silica columns are less affected by the retention factor because of their higher porosity resulting in larger peak volume than particulate columns, but they are less efficient at higher flow rate because of the presence of large macropores.
High-pH Columns: Why do chromatographers want to use high-pH mobile phases? High pH will sometimes provide improved selectivity between a target compound and impurities. In addition, basic compounds become neutral at high pH and often exhibit higher loading capacities compared to protonated species.
Silica gel has always had the limitation of dissolving at high pH and with a typical monolayer bonded phase, the upper pH limit is usually stated as pH 8. Over the years, to extend this upper limit, workers have coated the particle with polymer or a very high loading of bonded phase, used special phases to prevent hydroxide ion attack, used special organic buffers that impede the progress of attack, and so on. Silica-organic hybrids and polymer particles have been one solution to this pH limitation. The latter frequently have much lower efficiency than silica-based columns.
Recently, silica researchers started looking at other ways to extend the pH range of silica gel-based bonded phase particles. At HPLC 2014, posters of four different companies showed new proprietary silica-based products that exceed the regular pH 8 specification: AkzoNobel/Kromasil, Agilent Technologies, Advanced Chromatography Technologies (ACT), and Daiso. The Kromasil EternityXT material is based on a stable fully porous silica hybrid with organosilane reinforcement designed to work in a wider pH environment from 1 to 12. It is available in particle sizes from 1.9 to 10 µm, all with the same chemistry. Phases include C18 and phenyl-hexyl. The column can be rinsed with sodium hydroxide solution. The Agilent product is Poroshell 120 HPH and comes in C18 and C8 phases. It is a superficially porous particle with a 2.7-µm size with a recommended pH range of pH 3–11. Agilent uses a proprietary process to organically modify the Poroshell 120 silica support. The ACT UltraCore column comprises superficially porous particles with C18 and phenyl-hexyl bonded phases for reversed-phase applications. It uses Encapsulated Bonding Technology, which is designed to provide an extended pH range of 1.5–11.0. The fourth company, Daiso, a Japanese company, reported on two new base silicas featuring extended pH range: Peptisil for general peptide purification and Insusil for insulin purification. Both products are bulk silica designed for process scale LC.
Sub-2-µm Superficially Porous Particles: As reported earlier, SPP particles were still very much in vogue at HPLC 2014. Joe DeStefano of Advanced Materials Technology gave an interesting take on the future direction of UHPLC columns with a provocative title of his lecture "Are Sub-2-µm Superficially Porous Particles Needed for Small Molecule Separations?" DeStefano considered the tradeoffs between using SPPs in the 1.3–1.7-µm range (or even smaller) and the currently popular 2.5–2.7-µm sizes. As theory suggests, column efficiency is dramatically improved with smaller particles. However, the smallest SPP currently available does not provide the expected efficiency based on particle size, probably because of the difficulty of packing very small particles into homogeneous beds. In addition, most UHPLC and HPLC instruments are unable to maintain the high efficiencies of the columns because of band spreading in the extracolumn volumes of the connecting tubing and flow cells. Also, these smaller particle columns operate at extremely high pressures, require the use of more-specialized, more-expensive low-dispersion UHPLC systems, and may not be user friendly for routine operations. Smaller-particle SPP columns may be subject to more frictional heating, and columns with smaller internal diameters (≤3 mm) must be used to minimize this effect. Since smaller-porosity frits must be used to contain the packing, smaller-particle columns may be more subject to plugging. The practical answer is to have an SPP with a diameter between the smallest and those more typically being used today. DeStefano went on to recommend consideration of 2-µm SPP columns where the solid core would be 1.2 µm with the porous outer shell being 0.4 µm. He showed a series of van Deemter plots comparing sub-2-µm SPP and 1.7-µm porous particles to 2.0- and 2.7-µm SPP particles. The 2.0-µm, 50 mm × 2.1 mm SPP column gave 15,570 plates when operated with a flow rate of 0.5 mL/min and showed the best performance in terms of plates/bar compared to sub-2-µm columns.
In his keynote lecture, Professor Yukui Zhang of the Chinese Academy of Sciences in Dalian, China, showed some results on a 1.9-µm core–shell particle that was synthesized in his group's laboratory. Rather than the layer-by-layer method used in current technologies, they used a three-step method starting with a narrow particle size distribution (1.56 ± 0.06 µm) nonporous silica particle prepared by a modified, seeded growth method, a mesoporous shell formation by a one-pot template dissolution and redeposition strategy, and finally a pore size expansion via acid refluxing (HCl treatment). By such a strategy, core–shell materials with pore channels perpendicular to the particle surface, adjustable particle size, controlled shell thickness, tailored pore diameter, and high surface area could be obtained. The final material was derivatized for reversed-phase and chiral chromatography. The C18 phase displayed 211,300 plates/m for naphthalene. A wider pore version (1.5-nm pore size) was used to separate intact proteins. In the same presentation, Zhang also reported on their work on organic-silica hybrid monoliths.
Monodisperse Porous Silica Columns: Recently, with the emphasis on SPP and their narrow particle size distribution giving greatly increased performance, the idea of narrow particle size distribution totally porous particles for increased performance has arisen, especially since a new commercial product (Titan, Sigma Aldrich/Supelco) has been introduced. In a recent special issue of LCGC North America (8), Richard Henry addressed the issue of the impact of particle-size distribution on HPLC column performance. He found that a narrow distribution of porous particles did show better performance as evidenced with a lower reduced plate height with a lower pressure drop than a similar particle size with a wider particle size distribution. At HPLC 2014, Nagae and Tsukamoto of ChromaNik Technologies presented a poster where they too examined the effect of monodispersity on performance of reversed-phase packings prepared from similar silica starting materials. They compared a conventional 3.19 µm totally porous particle (D90/D10 = 1.48) to a 2.81-µm monodisperse totally porous particle (D90/D10 = 1.09) and a 2.78 µm core–shell particle (D90/D10 = 1.11). Reduced plate heights were 2.57, 2.22, and 1.75, respectively, indicating that the narrow distribution porous particle gave about 16% better efficiency than the conventional wider distribution porous particle. However, in their studies the core–shell particle outperformed the porous particles by nearly 50% on a reduced plate height basis.
HILIC: David McCalley of the University of the West of England can always be counted upon to deliver a clear, informative lecture and his keynote oral presentation at HPLC 2014 was no exception. He talked about new developments in HILIC. Table 2 shows the relative strength of HILIC in mode usage whose remarkable growth shown here agrees with a recent survey of LCGC readers (9). The mechanism of HILIC separations is quite complex, involving a mixture of partition of solutes into the water layer on the stationary phase surface, adsorption onto polar surface groups, and ionic interactions with the silica matrix for those particles based on that medium. In the present paper, David and colleagues further investigated the mechanism of HILIC, studying the effect of buffer composition on retention and peak shape of acidic, basic, and neutral solutes on a variety of columns — bare silica, zwitterionic, amide, and silica hydride. Buffers investigated included 0.1% formic acid (v/v), 5 mM ammonium formate (pH 3.0), and 0.1% phosphoric acid (v/v). Investigating a 90:10 (v/v) mixture of acetonitrile and the three buffers and measuring retention of the test compounds, he found that for the ammonium formate buffer, there was relatively low retention of neutrals and acidics but a higher retention of basic solutes, probably because of ionic interactions. For the formic acid buffer, there was an even higher retention of basic compounds compared to the ammonium formate buffer, perhaps because of the absence of competition from ammonium cations. For the phosphoric acid, ionic interactions couldn't be suppressed even at the low pH given by this acid. Formic acid gave poor efficiency for ionizable solutes with all types of silica-based HILIC columns in part because of the low ionic strength of the acid, which is exacerbated by the high concentration of acetonitrile in the mobile phase. Salt buffers like ammonium formate have a higher ionic strength and may also promote formation of the water layer on the column surface. Further evidence for ionic interactions was shown by comparing retention of the base pyridine and (neutral) uridine, which have similar hydrophilicity. However, pyridine is much more strongly retained. Consideration of the pH in the aqueous–organic mobile phase may be more appropriate in understanding retention.
Surprisingly, the silica hydride–based column gave similar selectivity to the silica column and a higher retention of cations just like bare silica columns.
As a side note, McCalley investigated the use of charged aerosol detection (CAD) and found it to be quite useful for HILIC separations, presumably because of the high content of organic solvent, and thus the ease of desolvation of solutes. The charged aerosol detector did show a nonlinear response curve for many solutes, but gave good sensitivity.
Column Packing Studies: Some researchers keep working on silica- and polymeric-monolith columns to realize their full potential, others continue to think about the next generation of conventional packed HPLC columns. For the latter group, the idea of decreased particle size immediately comes to mind, whether it is a totally porous particle or a superficially porous particle. The production techniques for such small particles, even submicrometer, are already available. The sizing could be a challenge for porous particles, although the process to make superficially porous particles is readily available. There are several obstacles facing researchers: Ensuring that the next generation of instruments will provide low enough dispersion to realize full efficiency of the columns at hand; the frictional heating that may arise from even smaller particles and higher pressures; the impact of higher pressures on physical and chemical properties of solvents and solutes; and the ability to pack particles effectively in all dimensions of tubing from microchannels to analytical scale. It should be noted in the latter case that many still consider HPLC column packing to be an art (5).
To that end, Jim Jorgenson's group at the University of North Carolina has begun a series of studies looking at optimized procedures to pack smaller particles (sub-2-µm) routinely and reproducibly. At HPLC 2014, he reported on some early results in their attempt to understand the slurry packing of capillary LC columns. Capillary columns, of course, would be best for heat dissipation, but present a bigger challenge than larger bore column formats. In their previous studies, they had success in packing nonporous reversed-phase packings as small as 0.9-µm but in the case of porous particles, column efficiency tended to decline as the particle diameter decreased, especially below 1.5-µm. They estimated that for small molecules, with operating pressures in the 1000–4000 bar range, particles of around 1-µm in size should be close to optimum.
In earlier work in their laboratories along with that of Ulrich Tallarek at Philipps-Universitat in Marburg, Germany, they considered packing morphology and separation efficiency of low-aspect-ratio capillary UHPLC columns. They combined chromatographic studies with confocal laser scanning microscopy to examine packing structure, including transcolumn porosity heterogeneity (6). The studies correlated structural features of the packing, such as an increased number of packing voids, with decreased efficiency. They investigated the effect of slurry concentration: 5, 10, 20 mg/mL all the way up to 100 mg/mL and found that higher slurry concentrations and rapid packing gave them higher numbers of packed bed voids while low slurry concentrations and slower packing gave more particle size segregation. They figured that a balance in concentration must be achieved to minimize the two types of bed heterogeneities. In considering bridged ethyl hybrid (BEH) particles between 1.4- and 1.9-µm, they found that for a given length of column they needed a different intermediate slurry concentration to produce the best performance. For example, they found that for a 34 cm × 75 µm capillary packed with fully porous 1.4-µm BEH particles that an optimum slurry concentration (lowest H value) was 20 mg/mL. When they tried to pack a 1-m capillary column with a 20-mg/mL slurry of 1.4-µm BEH particles, the results weren't conclusive. However, when they broke the column into three approximately 30-cm sections, the outlet section (that is, the first packed segment) of the column performed not only better than the other two sections, but also as expected given the initial slurry concentration study. Work is continuing on optimum slurry packing techniques for small particles and capillary columns.
Method Translation and Related Parameters
Since the advent of UHPLC and the myriad instruments with high pressure capability have been on the market, many laboratories are now looking to convert older methods to the newer instrument platforms. Method transfer should be a relatively painless exercise since all one has to do is to get a column with the same chemistry but with a different column particle size and perhaps a different column length and internal diameter. Wrong! There are a number of parameters that have to be addressed. Some aspects of method translation were covered in a tutorial and poster papers. A poster paper by Karim Kassam of Advanced Chemistry Development entitled "Translations Between Differing Formats of Liquid Chromatography: Advantages, Principles, and Possible Pitfalls" gave some food for thought in making these translations. Assuming one has a column with the same chemistry hopefully by the same manufacturer, this eliminates one overwhelming parameter that has to be overcome. The paper outlined the key factors to LC method translation whether it is HPLC to UHPLC, HPLC to HPLC, or UHPLC to HPLC. They are: Dwell volume differences, incorrect dead volume estimations, differences in instrument design, differences in efficiency caused by extracolumn band broadening effects and peak overloading, and retention time shifts as a result of heat of friction and pressure. Dwell volume is the volume from where the solvents A and B first meet to the head of the column. Without going into a lot of detail, there are methods available to determine dwell volume for a given instrument, but the value must be stated for any method because application of a fixed gradient for two instruments with different dwell volumes will result in a totally different chromatogram. The dead volume of a column is the volume to elute an unretained peak. When using different types of packed or monolithic columns, one must determine the dead volume by the injection of an unretained peak. In reversed-phase chromatography, something like uracil is frequently used. For more details, consult reference 7. Eduard Rogatsky of the Albert Einstein College of Medicine, New York, USA, gave a tutorial entitled "Dwell Volume: Hidden Aspects of UPLC Method Transfer". Rogatsky contends that different manufacturers measure dwell volume using different methodologies. He further states that there is no standard method to use. The physical volume of the system is not equal to the dwell volume (also called the gradient delay volume). The dwell volume will change with pressure if a pulse damper is part of the system. He recommends to measure the delay volume by using a water–acetone mixture in one pump and a UV detector to measure the absorbance change of the baseline. Make a step change (or linear gradient) and then take the midpoint (one-half the maximum absorbance) of the absorbance change. It all goes back to making sure that you measure the dwell volume the same way for both instruments when making a method translation and make sure you always state the dwell volume as part of the method.
Future HPLC Symposia
The next major symposium in this series, the 42nd Symposium, will be held 21–25 June 2015, in Geneva, Switzerland. The chairman of this meeting will be Professor Gérard Hopfgartner of the University of Geneva. For more information, go to www.hplc2015.org. On 21–25 September 2015, the series (#43) returns to China, this time being held in Beijing. The symposium chair will be Professor Guibin Jiang of the Chinese Academy of Sciences. You can learn about this meeting at the following website: www.hplc2015-beijing.org. If you can't make it to Switzerland or China, HPLC 2016 will return to San Francisco, California, USA, as it has every 10th year since HPLC 1986. The dates will be 19–24 June 2016, and the chair will be Professor Robert T. Kennedy of the University of Michigan. Bookmark all of these websites so that you can keep up on the latest happenings.
Acknowledgements
I would like to acknowledge the help of Laura Bush, Editorial Director of LCGC and Xiaoli Wang of Agilent Technologies for taking some notes on lectures that I couldn't attend. Thanks goes to Martin Gilar of Waters for supplying the great photos of the award winners pictured in this column.
Ronald E. Majors is the editor of "Column Watch," an analytical consultant, and a member of LCGC Asia Pacific's editorial advisory board. Direct correspondence about this column should be addressed to the editor-in-chief, Alasdair Matheson, at amatheson@advanstar.com
References
(1) R.E. Majors, LCGC Europe 27(4), 196–212 (2014).
(2) R.E. Majors, LCGC Europe 26(9), 505–516 (2013).
(3) R.E. Majors, LCGC Europe 25(9), 490–511 (2012).
(4) F. Svec and J.M.J. Fréchet, Anal. Chem. 64, 820 (1992).
(5) J.J. Kirkland and J.J. Destefano, J. Chromatogr. A 1126, 50–57 (2006).
(6) S. Bruns, J.P. Grinias, L.E. Blue, J.W. Jorgenson, and U. Tallarek, Anal. Chem. 84, 4496 (2012).
(7) P. Peterson, M.R.Euerby, and M.A. James, LCGC North Am. in press (2014).
(8) R.A. Henry, LCGC North Am. 32(s4), 12–19 (2014).
(9) R.E. Majors, LCGC North Am. 30(1), 20–34 (2012).
Best of the Week: Food Analysis, Chemical Migration in Plastic Bottles, STEM Researcher of the Year
December 20th 2024Top articles published this week include the launch of our “From Lab to Table” content series, a Q&A interview about using liquid chromatography–high-resolution mass spectrometry (LC–HRMS) to assess chemical hazards in plastic bottles, and a piece recognizing Brett Paull for being named Tasmanian STEM Researcher of the Year.
Using LC-MS/MS to Measure Testosterone in Dried Blood Spots
December 19th 2024Testosterone measurements are typically performed using serum or plasma, but this presents several logistical challenges, especially for sample collection, storage, and transport. In a recently published article, Yehudah Gruenstein of the University of Miami explored key insights gained from dried blood spot assay validation for testosterone measurement.
Determination of Pharmaceuticals by Capillary HPLC-MS/MS (Dec 2024)
December 19th 2024This application note demonstrates the use of a compact portable capillary liquid chromatograph, the Axcend Focus LC, coupled to an Agilent Ultivo triple quadrupole mass spectrometer for quantitative analysis of pharmaceutical drugs in model aqueous samples.
