High Temperature Analysis of Nine Common Pharmaceuticals Using Sub-2 µm ZirChrom-PBD
In this application note we examine the effect of temperature on a sub-2 µm zirconia-based phase for the analysis of nine common pharmaceuticals.
In this application note we examine the effect of temperature on a sub-2 μm zirconia-based phase for the analysis of nine common pharmaceuticals. Reducing the particle size and increasing the temperature increases both the efficiency and speed of separation without sacrificing resolution or column stability.
The chromatographer's ability to make practical use of the promised efficiency, and thus speed, benefits of sub-2 μm particles has long been hindered by the need for specialized high-pressure tolerant instrumentation. The superior selectivity and stability of ZirChrom® -PBD enables a much wider temperature (up to 150 °C) and pH (pH 1–14) range for method development. Elevated temperature speeds separations through the following three main effects (1). First, higher temperature increases the diffusion rate of analytes minimizing any losses in efficiency at higher flow rates (2). Second, at elevated temperature, the kinetics of the faster interactions between the analytes and stationary phase will lower the overall analysis time; often reducing or eliminating peak tailing. Finally, the viscosity of the mobile phase is decreased, enabling higher flow rates with existing equipment without increasing backpressure.
The decrease in mobile phase viscosity provided by high temperature is especially important for method development with sub-2 μm particles as it helps to overcome the higher back pressures inherent in small particle HPLC and allows the average user to take advantage of the increased efficiency provided by the smaller particles without the use of specialized UHPLC instrumentation.
Experimental
Nine pharmaceuticals were separated using a ZirChrom® -PBD column. The separation conditions were as follows:
Columns: Sub-2 μm ZirChrom® -PBD, 50 mm × 4.6 mm i.d. (Part Number: ZR03-0546-1.9)
Mobile Phase: 22/78 Acetonitrile/20mM Potassium Phosphate, pH 12.0
Injection Vol.: 2 ml
Temperature: 75 °C
Back Pressure: 246 bar
Flow Rate: 2.5 mL/min
Detection: UV at 254 nm
Figure 1 shows the separation of nine pharmaceutical compounds at 75 °C on a sub-2 μm ZirChrom® -PBD column. The instrument used in the analysis was a very basic commercially available HPLC system with minimal modification to eliminate as much void volume in the system as possible. As shown in Figure 1, the increase in temperature allowed an increase in flow rate, reducing the analysis time while keeping back pressure well below the 400 bar operating limit for standard HPLC equipment.
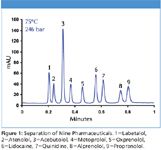
Figure 1
This method can be tailored to your specific application needs. ZirChrom technical support can help to optimize and transfer this method to your site. Please contact ZirChrom technical support at 1-866-STABLE-1 or support@zirchrom.com for details.
References
(1) Antia, F.; Horvath, C. J. Chrom. 435, 1–15 (1988).
(2) Li, J.W; Carr, P.W. Anal. Chem. 69(5), 837–843 (1997).

ZirChrom Separations, Inc.
617 Pierce Street, Anoka, MN 55303
tel. 1-866-STABLE-1;
Email: support@zirchrom.com
Website: www.zirchrom.com

SEC-MALS of Antibody Therapeutics—A Robust Method for In-Depth Sample Characterization
June 1st 2022Monoclonal antibodies (mAbs) are effective therapeutics for cancers, auto-immune diseases, viral infections, and other diseases. Recent developments in antibody therapeutics aim to add more specific binding regions (bi- and multi-specificity) to increase their effectiveness and/or to downsize the molecule to the specific binding regions (for example, scFv or Fab fragment) to achieve better penetration of the tissue. As the molecule gets more complex, the possible high and low molecular weight (H/LMW) impurities become more complex, too. In order to accurately analyze the various species, more advanced detection than ultraviolet (UV) is required to characterize a mAb sample.















