The Greening of the Chromatography Laboratory
How to measure a method's 'greenness' and examples of green chromatography in action.
The green chemistry movement has been evolving for well over a decade and this approach is starting to filter down to the chromatography laboratory. In this installment of "Sample Prep Perspectives," columnist Ron Majors and guest co-author, Douglas Raynie, discuss how to measure a method's "greenness" as well as describing examples of green chromatography in action.
For well over a decade, the green chemistry movement has been exploring ways to reduce the risks of chemical exposure to humans and the environment. There are many "green" movements throughout the world. A few chemists recognized fairly early that chemistry in many subdisciplines had great potential to reduce their environmental impact. One of those chemists, Dr Joseph Breen of the United States Environmental Protection Agency (EPA), was a visionary of green chemistry in the 1990s. Dr Breen founded the Green Chemistry Institute (GCI), now run by the American Chemical Society (ACS), and was its first director. The ACS GCI promotes green chemistry through information dissemination; research and fellowships; participation in conferences, workshops and symposia; international outreach; industrial partnership; awards and recognition; and education. In the United States, interest in green chemistry began in earnest with passage of the Pollution Prevention Act of 1990. In 1991, the EPA then established its own green chemistry programme under Dr Paul T. Anastas.
More recently, the ACS GCI formed a Pharmaceutical Roundtable whose mission was to catalyse green chemistry and green engineering in practice in the global pharmaceutical industry. Currently, a number of the largest pharmaceutical companies are members. Other ACS Roundtables addressing green chemistry have been or are being established. Several national and international green chemistry awards have been established. In the United States, the EPA's Presidential Green Chemistry Challenge Awards are presented to industry, small businesses and academia that recognize and promote innovative chemical technologies that prevent pollution and have broad applicability in industry. In 2009, the CEM Corporation (Matthews, North Carolina, USA) received this award for its Sprint Rapid Protein Analyzer, which measures protein content, such as in food (baby formula, pet food, and so forth) and distinguishes between melamine and protein, unlike the common Kjeldahl method. This was the first Challenge Award given to a company for an analytical instrument or technique. The ACS itself presents several awards, including one for affordable green chemistry sponsored by Dow Chemical (Midland, Michigan, USA) that recognizes global scientific discoveries that deliver cost-effective and environmentally friendly products and processes. Outside the United States, substantial awards for developments in green chemistry have been given by governments and chemical societies in Australia, Canada, Italy, Japan and the United Kingdom. On the education front, a large number of universities and colleges throughout the world have established centres for green chemistry and a few provide academic degrees in green chemistry.
What is Green Chemistry?
Simply stated, green chemistry reduces or eliminates the use or generation of hazardous substances from chemical products and processes. Green chemistry improves upon all types of chemical products and processes by reducing impacts on human health and the environment relative to competing technologies. Green chemistry technologies encompass all types of chemical processes, including synthesis, catalysis, reaction conditions, separations, analysis and monitoring. A green chemistry technology can involve implementing incremental improvements at any stage. It can, for example, substitute a greener feedstock, reagent, catalyst or solvent in an existing synthetic pathway. A green chemistry technology also can involve substituting an improved product or entire synthetic pathway. Ideally, a green chemistry technology incorporates the principles of green chemistry at the earliest design stages of a new product or process. Benefits to human health and environment may occur at any point in a technology's lifecycle: extraction, synthesis, use and ultimate fate.
Table 1 shows the 12 principles of green chemistry that provides a framework for scientists and engineers to use when designing new materials, products, processes and systems.1 In using these principles not all of them have to be applied simultaneously but should focus one's thinking in terms of sustainable design criteria. Three themes dominate the 12 principles: waste, hazard (health, environmental and safety) and energy. Most of the applications of green chemistry involve new or modified synthetic routes and catalysis studies. Not many published studies have involved green analytical chemistry.2

Table 1: 12 principles of green chemistry*.
What is Green Analytical Chemistry?
Many, but not all, of the 12 principles of green chemistry can be directly applied to analytical chemistry. Of those depicted in Table 1, numbers 1, 5, 6, 8, 11 and 12 are those principles considered to be most relevant to the analyst.2 Most applications of green chemistry in the analytical world involve designing analytical methods that reduce or eliminate hazardous substances. These substances can either be used in or generated by the method. Unfortunately, in the past, many environmental analytical methods required the use of large amounts of hazardous chemicals for sample preparation and analysis. For example, many liquid–liquid extraction methods used copious amounts of chlorinated solvents, such as methylene chloride. Before analysis, the solvent had to be disposed of by evaporation in the fume hood. Thus, toxic vapours were released to the atmosphere. So even early environmental analytical methods were not considered to be very green.
So, the goal of green analytical chemistry is to use procedures that generate less hazardous waste, are safer to use, and are more benign to the environment. Because there is much history in analytical method development and many validated methods that are currently in use, the ease of converting these methods to green analytical chemistry is easier said than done. Nevertheless, when developing new analytical methods, consideration of the "green" aspects should be taken into account. Reference texts on green analytical chemistry3,4 have appeared in the past few months and should help to better educate current and future analysts on the development of more ecofriendly methods.
What About Green Chromatography?
In the past, chromatographers gave little thought to the "green" aspects of their chromatographic methods. Only when regulatory or safety issues arose, such as the use of chlorinated solvents and certain aromatic solvents (for example, benzene), did workers consider substituting new, less toxic solvents in their method development schemes. The 2008–2009 acetonitrile shortage caused a lot of commotion and chromatographers started thinking about minimizing the use of this popular organic modifier for reversed-phase high performance liquid chromatography (RP HPLC). In 2009, one of us (REM) wrote a lengthy article in LCGC North America discussing ways to counteract the acetonitrile dilemma.5 Table 2 summarizes the important elements of this article. To get the complete story, please see reference 5. Unfortunately, after the acetonitrile shortage was over and prices returned to normal (although they're still high), many returned to their previous ways. Nevertheless, the shortage did bring attention to a potential problem that could reappear with another solvent crisis. Of course, the driving force for the reduction of acetonitrile usage at that time was cost and availability — not necessarily ecofriendliness.
Table 2: Methods to reduce acetonitrile use*.
Without addressing the use of hazardous and flammable solvents and chemicals used in sample preparation, the use of copious amounts of solvent as the mobile phase in RP LC analysis represents the single biggest area where the greening of the chromatography laboratory could be affected. A typical analytical liquid chromatograph can go through 1 L of organic solvent every day and even more if automation allows it to run overnight. The most popular modifier solvent for RP LC is acetonitrile, and its moderate toxicity properties are well known. Substitution of acetonitrile as an organic modifier in reversed-phase LC has been discussed in detail.5 Nevertheless, most workers in the field prefer to stay with their time-proven mobile phase systems and will probably react only when their company or institute makes a stronger commitment to the greening of their laboratories. The increasing use of hydrophilic interaction liquid chromatography (HILIC) means that more acetonitrile will be used in the mobile phase unless alternative greener solvents are found (see later for one such example). The HILIC separation technique uses large amounts of acetonitrile (upwards of 80%) in the aqueous mobile phase; it is preferred by mass spectrometrists because ion suppression effects are less than those observed in reversed-phase LC.
One of the immediate approaches to reduced solvent usage in chromatography laboratories is to decrease the column volume by shortening the length or decreasing the internal diameter (Table 2). The increasing use of mass spectrometry (MS) as a detection principle for HPLC actually is a "greener" technique than LC–UV because most mass spectrometrists prefer to use smaller internal diameter columns, 2 mm and under, due to the increased sensitivity of the instrument and inability to cope with the larger amounts of solvent vapour from larger internal diameter columns, 4.6 mm i.d. and over. A typical 2.0 mm i.d. column uses one-fifth of the solvent that a 4.6 mm i.d. column uses when run at the same linear velocity; a 1.0 mm microbore column uses 1/20 of the solvent consumed with a 4.6 mm i.d. column. So, obviously the next step is further miniaturization, and this movement is already occurring. Capillary columns with internal diameters in the 100–300 μm range have been available for some time and a modern generation of instruments optimized to run with them under ultrahigh-pressure liquid chromatography (UHPLC) conditions is already available or right around the corner. Further miniaturization to nanocolumns has also occurred; these columns have internal diameters of 50–75 μm and include HPLC-chip columns that interface directly to a nanospray LC–MS system.6 The greenness of the nanoLC technique is only valid with the use of nanoflow-rate (microfluidic) pumps because a split-flow HPLC system still wastes most of the solvent. With "true" nanosystems, solvent usage is almost miniscule (in the range of a few tens of millilitres each month), already a "green" chromatographic approach. Miniaturization not only cuts down on mobile phase solvent use but also minimizes sample mass requirements and sample solvent usage. Such low usage opens up the use of exotic or expensive solvents as HPLC mobile phases that may be greener in their chemical characteristics.
How Does One Measure the Greenness of an Analytical Technique?
There are a great many factors that must be considered when developing an analytical measurement. Among the most important criteria are the method's sensitivity, selectivity, linearity, limit of detection (LOD), limit of quantification (LOQ), speed, accuracy and precision. The nature of the analyte, the matrix and the method of generating a signal proportional to the concentration of all analytes come into play when developing a successful analytical method. Literature is scarce on assessing an analytical method with respect to its "greenness." Some reports have been selective, considering only one aspect of a method. A comprehensive assessment of a chemical method requires consideration of all parts of the analytical method. Consideration of the potential greenness of a method should be thought about during the method development stage alongside the other important criteria. Ideally, methods that need little or no sample preparation, use few or a minimal number of chemical reagents, and are conducted in a totally aqueous environment would be preferred over a method that requires sample pretreatment such as acid digestion, solvent extraction, evaporation, reconstitution, derivatization and flash chromatography. Most conventional LC methods do not fit into the former category because both the sample preparation and the separation involve the use of organic solvents, sometimes in copious amounts.

Table 3: Greenness profile criteria for assessing greenness of method*.
Can the greenness of an analytical method be quantified? A couple of approaches to quantify the greenness of an analytical method have been proposed. The ACS GCI, working in collaboration with a panel of environmental analytical experts, has developed greenness criteria for environmental methods. The criteria have been applied to the National Environmental Methods Index (NEMI), a free internet-searchable database of environmental methods.7 The database contains information such as method summaries, metadata and links to full methods. Greenness profiles have been added to the database so that one can look at a method's rating and see how green it is. There are currently nearly 1000 methods that have been added to the NEMI database. The profile criteria for these methods were based on four key terms: PBT (persistent, bioaccumulative and toxic), hazardous, corrosive and waste. The 12 principles of green chemistry (Table 1) were used as a guideline in establishing this profile. Table 3 provides more information on the profile criteria. To make it easy to peruse the variety of methods, a greenness profile symbol was developed [see Figure 1(a)]. Each quadrant is either "green" or "blank" depending on the method fit to that particular criterion. Thus, by observing the overall profile, an analyst can quickly see if one method is less green or greener than another method. This approach is especially helpful when a variety of approved methods exists and one wants to locate the greenest method.
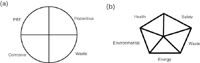
Figure 1: (a) Greenness profile symbol from the NEMI.gov greenness profile and (b) setup of the green assessment summary symbol.
Here's a simple example of the use of the assessment to find a green analytical method: We want to find an EPA regulatory method for the pesticide aldrin in water with a detection limit of less than 0.2 μg/L and a relative standard deviation (RSD) of less than 20%. A search of the NEMI database finds two acceptable methods: EPA Method 525.2 and EPA Method 505. Method 505 involves the analysis of organohalide pesticides and commercial polychlorinated biphenyl products in water by microextraction and gas chromatography (GC), and Method 525.2 involves the determination of organic compounds in drinking water by liquid–solid extraction (LSE) and capillary column GC–MS. Figure 2 shows the greenness profiles of the two methods. Note that Method 505 has three of four quadrants filled in green while Method 525.2 has only the PBT quadrant showing green. Thus, 505 is the greener of the two methods. Why is this? Method 525.2 uses ethyl acetate, methylene chloride, and methanol to extract the pesticides captured by solid-phase extraction (SPE) from 1 L of water to which hydrochloric acid is added to reduce the pH to greater than 2; more than 50 g of waste is generated. Method 505 uses only 2 mL of hexane to extract 35 mL of water with no pH adjustment, so less than 50 g of waste is generated. Therefore, only the hazardous quadrant is left uncoloured because hexane is on the TRI list. For more detailed information on the applications of the greenness profiles to environmental methods in the NEMI database, consult reference 2.
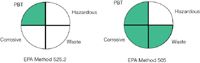
Figure 2: Comparisons of greenness profile of two EPA methods for aldrin in water.
Some workers have investigated the greenness of a portion of the analytical methodology. For example, Capello, Fisher and Hungerbuhler10 have developed a system for assessing the greenness of solvents, taking into account physical, chemical, toxicological, environmental and lifecycle properties of a selected group of solvents and solvent mixtures. But even this approach failed to consider all aspects of the solvent greenness with respect to the overall chemical processes. It should be noted that while toxicological data for pure solvents may be known, properties of solvent mixtures may not be additive from the individual solvents. For example, hexane–acetone mixtures are much more toxic than either hexane or acetone alone. The problem is that toxicity data are not well known for individual chemicals, let alone mixtures.
More recently, a simple system has been proposed for evaluating and comparing chemical methods for greenness.11 The system is patterned after the NEMI greenness profile but includes energy as a criterion and increases the range that is available within a particular criterion, allowing additional discrimination when comparing methods.11 The hazard category was divided into health, safety and environmental hazards. The addition of energy as a criterion is important due to the high reliance on non-renewable resources (fossil fuels) for electrical energy production. This criterion takes into account the amount of energy consumed by instrumentation and equipment used in the analytical method. In this assessment, the length of time that is required for an instrument and peripheral equipment comes into play and the energy used is expressed in kilowatt-hours for a typical run. For this criterion, greenness scores are given based on power consumed. To simplify comparative assessments, a Green Assessment Summary figure was implemented [see Figure 1(b)]. Using this newer assessment method, a number of current methods (EPA and others) for the analysis of polynuclear aromatic hydrocarbons in water were compared.9 A report on the detailed assessment for these methods is beyond the scope of this article but the Green Assessment Summary approach indicated that the assessment does offer a finer range of discrimination than the previous assessment approach for the determination of an analytical method's greenness.
The issue of whether or not to include energy requirements should figure into the assessment of a green method is still open to debate. For example, based on the chemicals used in supercritical-fluid chromatography (SFC), one would consider SFC to be a greener chromatographic method than HPLC. One study12 compared preparative-scale SFC with preparative-scale LC in the pharmaceutical industry using a life-cycle analysis approach. When boundary conditions were set at the process or plant levels, LC was found to consume 26–29% more resources than the SFC approach. However, when a Cumulative Exergy Extracted from the Natural Environment method — a broader energy conservation approach — was used, preparative SFC required 34% more resources. Thus, when the total power requirements needed to generate a supercritical fluid are compared with using a pump to move liquid through a column, all of a sudden SFC may not be the right choice for purification. However, as with life-cycle analyses, key variables include the establishment of boundary conditions and the assumptions that were made. This analysis shows that establishing what is truly green is not straightforward.
Some Examples of the Greening of the Chromatography Laboratory
Currently, the most widely used conventional column dimensions (4.6 mm i.d.) and favoured mobile phase (acetonitrile) used in LC do not pass any greenness criteria, especially for waste. Only through the use of smaller column volumes can the amount of solvent be reduced. However, capillary and nano-scale columns are still not the norm. So, workers who are interested in making their methods greener have investigated the following aspects:
- Alternative solvents that are more green (or are perceived to be more green).
- Approaches that reduce the amount of organic solvent in the mobile phase, several of which have been covered5 and are briefly outlined in Table 2.
Use of Alternative Solvents That Are More Green
Recently, SFC has seen a renewed interest, especially in the pharmaceutical industry, for chiral and preparative separations but also for its greenness. The most popular SFC mobile phase is carbon dioxide. It is considered a greenhouse gas that requires energy to compress it after recovery from fermentation processes and more energy to compress it for SFC use, as discussed earlier. However, carbon dioxide is not considered to be a toxic chemical and has many advantages as a chromatographic mobile phase. To increase its polarity, the carbon dioxide is doped with polar solvents such as methanol or tetrahydrofuran at the 5–20% level, but the overall quantity of organic solvent consumed is greatly reduced compared to HPLC. An earlier publication5 discussed the role of SFC as a greener chromatographic separation method, and it won't be further discussed here. For those interested in the exploring the greenness of SFC, Sandra and coworkers13 have recently discussed this area in detail.
A recent article provided a novel approach to green chromatography. The system of Fogwill and Thurbide14 of the University of Calgary (Alberta, Canada) uses water (saturated with carbon dioxide) as a stationary phase and carbon dioxide (saturated with water) as the mobile phase. Because the two solvents have little miscibility, conditions can be attained that create a stationary phase of water lining the inside of an uncoated stainless steel capillary. Because altering the temperature and pressure can change both the density of the mobile phase and the polarity of the stationary phase, adjusting these experimental parameters offers good flexibility for optimizing separations and allows different programmed gradient separation conditions. The system showed good retention time reproducibility, peak symmetry and sample capacity. Polar compounds (alcohols, carboxylic acids and phenols) showed good peak shape. Some applications included the determination of ethanol in alcoholic beverages; the organic-free solvent system allows the use of a flame ionization detector as is used in GC.
Pfizer (Sandwich, Kent, United Kingdom) is a pharmaceutical company that has seriously addressed the green chemistry issue.15 One specific approach that enabled the company's researchers to decrease the use of hazardous solvents was to use a solvent selection guide.16 Solvents are classified in decreasing order of greenness based on several properties. For example, water is on the top of the listing followed by acetone and ethanol, and benzene and carbon tetrachloride are on the bottom. Presumably, a chemist looking for a solvent to use could, in addition to solubility, polarity and other selection criteria, also choose a solvent higher on the green end of the solvent selection guide to develop a greener method. A solvent replacement table was generated that a researcher could use to replace a less green solvent with a more green solvent or solvent mixture of equivalent solvent strength. By this approach, they were able to decrease the use of dichloromethane in the sample preparation and chromatography laboratories by 50% in a span of four years.
In the chromatography group at Pfizer, 95% of all acetonitrile on the site was used for analytical purposes.17 Not only was the purchase of this solvent expensive, but many thousands of litres of laboratory waste had to be carried off and disposed of by incineration every month, at additional cost. They embarked on a study to look at alternatives to the use of acetonitrile and HPLC [for example, SFC, normal-phase HPLC and process analytical technology (PAT)] and found that for some of their applications, ethanol could be substituted without a deleterious effect on the chromatography. Figure 3 shows a comparison of the former reversed-phase ion-pair method using an acetonitrile–ammonium acetate buffer [Figure 3(a)] and the new ion-pair method based on an ethanol–ammonium acetate buffer [Figure 3(b)]. The columns were slightly different in dimensions, type of particle and stationary phase, but the results for the ethanol-based mobile phase were acceptable. The group has applied this solvent conversion to several of their active pharmaceutical ingredient (API) processes. From an ecofriendly viewpoint, ethanol is highly desirable due to its low toxicity and the fact that it is derived from renewable sources.

Figure 3: Comparable separation by subsitution of acetonitrile with ethanol. (a) Column = 50 mm à 3.0 mm, 1.8 μm Zorbax Extend C18 (Agilent Technologies, Santa Clara, California); mobile phase A = 10 mM ammonium acetate; mobile phase B: acetonitrile; temperature = 50 °C, flow rate = 1.0 mL/min; sample: proprietary API and associated impurities. (b) Column = 50 mm à 4.6 mm, 2.7 μm Halo C18 (MacMod Analytical, Chadds Ford, Pennsylvania); mobile phase A = 10 mM ammonium acetate; mobile phase B = ethanol; temperature = 50 °C; flow-rate = 1.5 mL/min; sample = proprietary API and associated impurities.
Ionic liquids (ILs) have drawn increased attention in analytical chemistry in the past decade. These liquids are generally composed of large cation (often ammonium or phosphonium salt) and smaller inorganic anions such that the coordination (electrostatic attraction) between ions is somewhat weak. Hence, below 100 °C these ILs are liquids with low volatility, somewhat tunable viscosity and miscibility, and good temperature stability and electrolyte conductivity. Because of their low volatility the ILs are considered to be green solvents. However, some of the ILs are also toxic, their biodegradability is questionable, recycling is not always feasible, and they are expensive compared to other solvents. Although ILs have found use as GC stationary phases and as additives in HPLC at very low levels,18 their use as an organic solvent replacement in HPLC at high percentage levels has not been taken seriously. Their high viscosities and high UV cutoffs make them very unattractive.
Approaches that Reduce the Amount of Organic Solvent in the Mobile Phase
We've already talked about some approaches that reduce the amount of organic solvent mobile phase (Table 2). For example, using higher column temperatures reduces solvent viscosity and retention times, and thereby to achieve the same separation time, one can reduce the amount of organic solvent. Using shorter-chain alkyl phases and lightly loaded RP materials one can achieve the same retention as for long-chain alkyl phases and highly loaded RP materials by using a lower percentage of organic solvent. Of course, the use of pure water as a mobile phase has been demonstrated by working at temperatures in the range of 200 °C with specialized column packings capable of operation at these temperatures.19 The hydrophobicity of thermoresponsive polymers can be modified by temperature changes.20 Above the lower critical solution temperature, the polymers behave like conventional RP materials but without the need for organic modifiers; pure water is used as the mobile phase.
Some interesting new approaches for the reduction of organic solvent in the mobile phase are coming on the scene. Spanish scientists have devised a greener form of reversed-phase LC using cyclodextrins (CDs) as additives in the mobile phase in the separation of β-carbolines.21 Cyclodextrins are natural renewable compounds made from starch via enzymatic conversion and are totally biodegradable. A favourable consequence of the presence of CDs in the mobile phase was the reduction of the organic solvent-to-water ratio without a decrease in selectivity or resolution. Furthermore, it also allows the use of renewable solvents (methanol and ethanol) as the organic components and avoids the use of the more toxic acetonitrile. Using this technique, β-carbolines, naturally occurring alkaloids linked to neurological diseases, were determined in human blood serum. Analytical results were as good as noncyclodextrin-modified HPLC in terms of accuracy; sensitivity was actually enhanced by the presence of the cyclodextrins. The separation mechanism involved a CD–carboline association allowing for a reduction in retention compared to the absence of the CD in the mobile phase. Another alternative to reducing organic solvent consumption involves the use of micellar LC, an approach that combines conventional stationary phases with mobile phase and is modified by the presence of surfactants and reduced proportion of organic solvents.22 One drawback of this technique, however, is the tendency of the surfactants to become adsorbed on the stationary phase.
As mentioned earlier, HILIC is not considered to be a green chromatographic method because of the high concentration of the acetonitrile used in the mobile phase. Recently, workers from Belgium came up with a green HILIC-enhanced fluidity method that used the addition of high concentrations of carbon dioxide to a mobile phase composed of ethanol–ammonium formate/formic acid buffer that allows the replacement of the acetonitrile–ammonium formate/formic acid buffer.23 The analysts used a silica gel column to separate polar nucleobases with the HILIC-enhanced fluidity method in roughly the same time as the acetonitrile HILIC-enhanced fluidity method HPLC system.
Future of the Further Greening of the Chromatography Laboratory
The adoption of the 12 principles of green chemistry in the chromatography laboratory has been slow in developing. Besides ethical and ecological considerations, economics is a strong driving force in further movements in green chromatography. Many laboratories have standard ways of developing chromatographic methods and are using validated methods and standard operating procedures, and many chemists are stuck in their ways. Only when a top-down approach is demanded — as has been done at some of the major pharmaceutical companies, where the laboratory managers and bench chemists are measured on their contribution to the greenness aspect of their jobs — will laboratories more seriously consider reduction in the use of hazardous and copious amounts of organic solvents, and reduction in the use of toxic and corrosive chemical reagents, waste generation and use of power gobbling instrumentation. With respect to the latter consideration, energy requirements don't usually come into play in many analytical laboratories because the overall site budget includes power expenses and these costs are allocated across many departments in a typical company. Nevertheless, many instrument companies have been lowering the power requirements for their instrumentation, just as has taken place in the consumer sector.
Training of green chemistry at the grass roots level in educational institutions (high school and university level) will help to develop a new mindset for future analytical chemists and chromatographers.
Nevertheless, great strides are being made in incorporating green chemistry into analytical laboratories, and eventually these advances will roll over into chromatography laboratories. The advances will most likely be made in the development of new methods where opportunities to incorporate green chemistries will arise.
Douglas Raynie is research associate professor at South Dakota State University. His research interests include green chemistry, alternative solvents, sample preparation, high-resolution chromatography and bioprocessing in supercritical fluids. He earned his PhD in 1990 at Brigham Young University under the direction of Milton L. Lee.
Ron Majors is "Sample Prep Perspectives" Editor and is senior scientist, Columns and Supplies Division, Agilent Technologies, Wilmington, Delaware, and is a member of LCGC's editorial advisory board. Direct correspondence about this column to LCGC editor, Alasdair Matheson, Advanstar Communications (UK) Ltd, 4A Bridgegate Pavillion, Chester Business Park, Wrexham Road, Chester CH4 9QH, UK or e-mail amatheson@advanstar.com
References
1. P. T. Anastas and J.C. Warner, Green Chemistry: Theory and Practice (Oxford University Press, New York, 1998).
2. L.H. Keith, L.U. Gron and J.L. Young, Chem. Rev., 107, 2695–2708 (2007).
3. M. Koel and M. Kaljurand, Green Analytical Chemistry, (Royal Society of Chemistry, London, 2010).
4. M. de la Guardia and S. Armenta, Green Analytical Chemistry, Vol. 57: Theory and Practice (Elsevier, Amsterdam, 2010).
5. R.E. Majors, LCGC North America, 27(6), 458–471 (2009).
6. G.O. Staples et al., Anal. Chem., 82(2), 516–522 (2010).
7. www.nemi.gov
8. Emergency Planning and Community Right-to-Know Act, Section 313, Toxic Release Inventory (TRI), available at www.epa.gov/tri/chemical
9. Code of Federal Regulations, Title 40, Part 261, available at www.ecfr.gpoaccess.gov
10. C. Capello, U. Fisher and K. Hungerbuhler, Green Chem., 9, 927–934 (2007).
11. D. Raynie and J.L. Driver, 13th Green Chemistry and Engineering Conference, Washington, DC, 2009.
12. G. Van der Vorst et al., Green Chem., 11(7), 1007–1012 (2009).
13. P. Sandra et al., LCGC Europe, 23(8), 396–405 (2010).
14. M.O. Fogwill and K.B. Thurbide, Anal. Chem., 82, 10060–10067 (2010).
15. http://www.pfizer.com/responsibility/protecting_environment/green_chemistry.jsp
16. P.J. Dunn et al., Green Chem., 9, 31–36 (2008).
17. G.W. Sluggett et al., Green Analytical Chemistry, British Pharmaceutical Conference, Manchester, United Kingdom, Sept. 9, 2008.
18. S.A. Shamsi and N.D. Danielson, J. Sep. Sci., 30(11), 1729–1750 (2007).
19. Y. Yang, A.D. Jones and C.D. Eaton, Anal. Chem., 71, 3808–3813 (1999).
20. H. Kanazawa et al., Anal.Chem., 72(24), 5961–5966 (2000).
21. V. Gonzalez-Ruiz et al., Green Chem., 13, 115–116 (2011).
22. L. Zhu et al., Green Chem., 11, 132–137 (2009).
23. A.S. Pereira et al., J. Sep. Sci., 33, 834–837 (2010).
Study Explores Thin-Film Extraction of Biogenic Amines via HPLC-MS/MS
March 27th 2025Scientists from Tabriz University and the University of Tabriz explored cellulose acetate-UiO-66-COOH as an affordable coating sorbent for thin film extraction of biogenic amines from cheese and alcohol-free beverages using HPLC-MS/MS.
Multi-Step Preparative LC–MS Workflow for Peptide Purification
March 21st 2025This article introduces a multi-step preparative purification workflow for synthetic peptides using liquid chromatography–mass spectrometry (LC–MS). The process involves optimizing separation conditions, scaling-up, fractionating, and confirming purity and recovery, using a single LC–MS system. High purity and recovery rates for synthetic peptides such as parathormone (PTH) are achieved. The method allows efficient purification and accurate confirmation of peptide synthesis and is suitable for handling complex preparative purification tasks.






